Medical/Pharmaceuticals
Innovent Announces First Patient Dosed in a Phase 3 Clinical Trial (CLEAR) of Picankibart (Anti-IL23p19 Monoclonal Antibody) in Patients with Moderate-to-Severe Plaque Psoriasis
ROCKVILLE, Md. and SUZHOU, China, Feb. 16, 2023 /PRNewswire/ -- Innovent Biologics, Group. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology ...
Tetracore Announces USDA Purchase of ASF and FMD Diagnostic Test Kits
ROCKVILLE, Md., Feb. 16, 2023 /PRNewswire/ -- Tetracore, Inc. (Tetracore®) is pleased to announce its partnership with the United States Department of Agriculture's (USDA) Animal and Plant Health Inspection Service (APHIS) to provide a stock of PCR kits for both African Swine Fever (ASF) and Foo...
Bioved Pharmaceuticals, Inc. announces the launch of its proprietary Natural Products for Animal Health, with its Shwan Ayurved™ science
SAN JOSE, Calif., Feb. 16, 2023 /PRNewswire/ -- Bioved Pharmaceuticals, Inc. is pleased to announce the launch of its scientifically validated Natural Animal Health products from the science of Ayurveda. Bioved is a Discovery Platform for safe and effective plant-based nutraceuticals, dietary sup...
Renexxion Ireland Ltd. and Dr. Falk Pharma GmbH Announce Initiation of Phase II Study of naronapride in patients with gastroparesis
Randomized, double-blind, placebo-controlled study to evaluate efficacy of novel pan-GI prokinetic in patients with gastroparesis ROSCREA, Ireland and FREIBURG, Germany, Feb. 15, 2023 /PRNewswire/ -- Renexxion Ireland Limited, a private biopharmaceutical company committed to delivering innovativ...
Internationally Renowned Orthopaedic Surgeon Thought Leaders Join Formus Labs' Clinical Advisory Board
AUCKLAND, New Zealand, Feb. 15, 2023 /PRNewswire/ -- Formus Labs, creator of the first automated 3D AI-enabled pre-operative planner for joint replacement surgeries, today announces that arthroplasty experts DrJ. Bohannon Mason, Dr. Atul Kamath, and Dr. James A. Germano will join its clinical adv...
Kubota Tractor Corporation Implements Syncron For Streamlined Retail Inventory Management and Exceptional Dealer Network Service
Leading distributor of agricultural and construction machinery equipment selects Syncron Retail Inventory for optimized dealer inventory management that aligns aftermarket strategies with dealer networks for superior customer service. CHICAGO, Feb. 15, 2023 /PRNewswire/ -- Syncron today announce...
Flagship Biosciences Applauded by Frost & Sullivan for Changing Biomarker Analysis to Find Biomarkers that Aid Drug Development and Diagnostics
Digital pathology solutions are increasingly used in the preclinical and clinical development phases, particularly toxicologic pathology and clinical trials, speeding up the process and curtailing expenses. SAN ANTONIO, Feb. 15, 2023 /PRNewswire/ -- Frost & Sullivan assessed the artificial intel...
Henlius Announces U.S. FDA Acceptance of Biologics License Application for Proposed Biosimilar Trastuzumab HLX02
* The first Chinese biosimilar approved in both China and the EU, and potentially to be approved in the U.S. - * HLX02 (trastuzumab for injection, trade name in China: HANQUYOU; trade name inEurope: Zercepac®; trade names in Australia: Tuzucip® and Trastucip®) has been approved in more than 3...
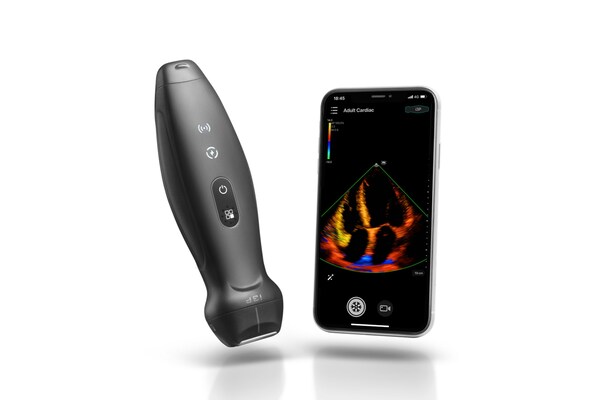
Mindray Revolutionizes the Way of Using Ultrasound with TE Air, Its First Wireless Handheld Ultrasound System
SHENZHEN, China, Feb. 15, 2023 /PRNewswire/ -- Mindray
GILEAD TO PRESENT DATA AT APASL 2023 ON EFFECTIVE MODELS FOR MONITORING AND TREATING HEPATITIS C AND EFFICACY OF ANTIVIRAL TREATMENTS FOR HIV-1 AND HBV-COINFECTED ADULTS
- Study recommends a simplified strategy for baseline testing and minimal monitoring for treatment of hepatitis C virus (HCV) infection with DAA Therapies - Review of implementation science projects toward HCV Elimination by 2030 in Asia proved effective in improving patient outcomes - Efficacy ...
Innovent Announces First Participant Dosed in Phase 2 Study of IBI311 (Anti-IGF-1R Monoclonal Antibody) in Patients with Thyroid Associated Ophthalmopathy
ROCKVILLE, Md. and SUZHOU, China, Feb. 15, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology an...
Groundbreaking clinical trial underway to improve vision in individuals with advanced Retinitis Pigmentosa (RP)
Putting Australian clinical research on the map globally, comes the ground-breaking first-in-human clinical trial for KIO-301. MELBOURNE, Australia, Feb. 15, 2023 /PRNewswire/ -- In collaboration with US-based Kiora Pharmaceuticals, Inc. (NASDAQ: KPRX) and The Royal Adelaide Hospital, leading Au...
Telix Collaboration on Next Generation Urologic-Oncology Theranostic Published in Journal of Nuclear Medicine
MELBOURNE, Australia, Feb. 15, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces the successful completion of its joint research project conducted with Heidelberg University Hospital (UKHD) under the Research Cooperation Agreement announced inFebrua...
Eurofins Discovery Collaborating with Invasight on Cancer Therapies
SAN DIEGO, Feb. 14, 2023 /PRNewswire/ -- Eurofins Discovery, the leading provider of products and services to the drug discovery industry, today announced its collaboration withInvasight, a Switzerland-based biotech developing cancer therapies. The collaboration focuses on advancing first-in-cla...
CJ Biomaterials Teams with Banila Co. to Develop New 100% Biobased Cosmetic Jar using Amorphous PHA
WOBURN, Mass., Feb. 14, 2023 /PRNewswire/ -- CJ Biomaterials, Inc., a division ofSouth Korea-based CJ CheilJedang and a primary producer of polyhydroxyalkanoates (PHAs), has produced the first cosmetic jar based on their amorphous PHA technology, under the tradename, PHACT™. The cosmetic jar was...
Metabolon to Offer Targeted Panels and Assays to Aid in Dysregulated Glucose Status and Related Research
Targeted metabolomics panels and assays provide absolute quantitation for advanced research into the relationship of metabolites to glucose metabolism MORRISVILLE, N.C., Feb. 14, 2023 /PRNewswire/ -- Metabolon, Inc., the global leader in providing metabolomics solutions that advance a wide variet...
Athachi Group showcases 'Trust Nature™' initiative to Union Ministers; The 'Trust Nature™' philosophy is akin to Yoga to the world
NEW DELHI, Feb. 14, 2023 /PRNewswire/ -- The senior management team of Athachi Group, a diversified business group headquartered in Palakkad, Kerala, with nature close to its heart across its operations inIndia and the UAE, met the Union Ministers recently in the capital and presented its 'Trust ...
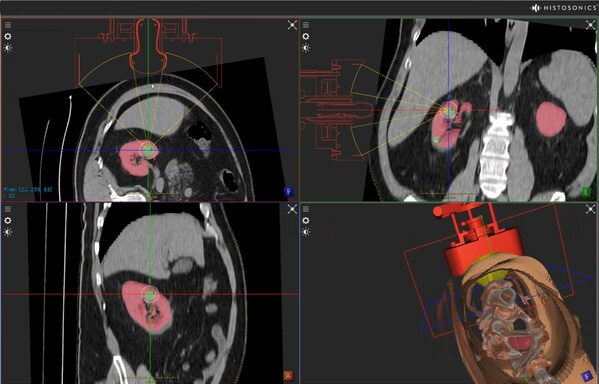
HistoSonics Announces FDA Approval of its Pivotal IDE #HOPE4KIDNEY Trial
Company to Utilize its New Edison™ System with Advanced Imaging Features for Use in Kidney Tumors MINNEAPOLIS, Feb. 13, 2023 /PRNewswire/ -- HistoSonics, (www.histosonics.com ), the developer of a non-invasive platform and novel sonic beam therapy called histotripsy, announced today the US Food a...
Point-of-Care Ultrasound Training in Under-Resourced Region Improves Physician Knowledge
Study of 100+ Patients Published in Journal of Radiology Nursing ROCKVILLE, Md., Feb. 13, 2023 /PRNewswire/ -- A study published for the March 2023 issue of Journal of Radiology Nursing demonstrates that a short training course in Point-of-Care Ultrasound (POCUS) can measurably improve physician...
U.S. Government and Novavax Extend Partnership, Securing Up to 1.5 Million Additional Doses of Novavax' COVID-19 Vaccine
* Modified agreement also includes the development of an updated vaccine in fall 2023 GAITHERSBURG, Md., Feb. 13, 2023 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious diseases, toda...
Week's Top Stories
Most Reposted
LONG AN INTERNATIONAL PORT JOINS 12TH PORTECH ASIA SUMMIT 2025 IN MALAYSIA
[Picked up by 335 media titles]
2025-01-18 03:30Hong Kong Airlines Takes Off to Australia's Gold Coast Bringing Popular Travel Option for the Chinese New Year
[Picked up by 308 media titles]
2025-01-18 17:00HARD ROCK HOTEL BALI ACHIEVES PRESTIGIOUS GSTC CERTIFICATION
[Picked up by 305 media titles]
2025-01-20 14:27dss⁺ Announces Strategic Changes to Executive Leadership Team in Asia Pacific Accelerating growth and impact for high-hazard industries in the region
[Picked up by 303 media titles]
2025-01-21 16:00Infobip Research Unveils Opportunities for Brands to Engage Customers with Rich Communication Services (RCS) across APAC
[Picked up by 288 media titles]
2025-01-20 21:09