Medical/Pharmaceuticals
Switching to investigational once-weekly insulin icodec from other basal insulins demonstrated to be efficacious and well-tolerated for people with type 2 diabetes in phase 2 trial
BAGSVAERD, Denmark, Sept. 22, 2020 /PRNewswire/ -- Today, Novo Nordisk announced results from three phase 2 clinical trials for insulin icodec, an investigational once-weekly basal insulin analogue, which were presented during the 56th European Association for the Study of Diabetes (EASD) Annual ...
New Study Suggests GlycoMark® May Identify Highest Risk Patients with Prediabetes
RALEIGH, North Carolina, Sept. 22, 2020 /PRNewswire/ -- According to new data presented at the 56th European Association for the Study of Diabetes (EASD) annual meeting, the GlycoMark blood test reflects beta-cell functional mass, which is important in identifying prediabetic individuals with the...
TCM Lianhua Qingwen Capsule approved for registration in Mauritius
SHIJIAZHUANG, China, Sept. 22, 2020 /PRNewswire/ -- Yiling Pharmaceutical Co., Ltd. (SHE: 002603) announced on Tuesday that it has received a registration approval issued by the Ministry of Health and Wellness ofMauritius, indicating Lianhua Qingwen Capsule has been registered in accordance with ...
Kazia Enters Clinical Collaboration With Dana-farber Cancer Institute For Primary CNS Lymphoma
SYDNEY, Sept. 22, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that it has entered into a collaboration with Dana-Farber Cancer Institute (DFCI) inthe United States, to investigate the use of...
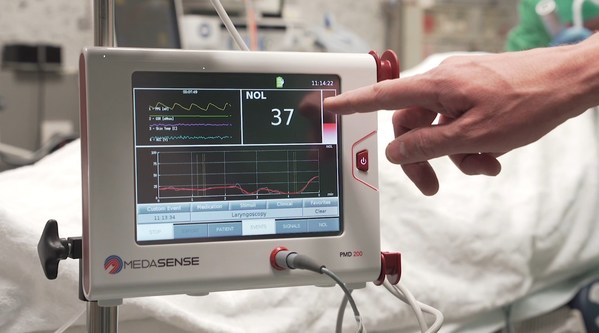
Israeli Pain Monitoring Startup Medasense Raises $18M in Series C Round
Accelerating Commercialization of its Novel Pain Monitoring Solutions RAMAT GAN, Israel, Sept. 22, 2020 /PRNewswire/ -- Medasense Biometrics Ltd., developer of the NOL technology for pain-response monitoring, today announced that it has raised$18M in a series C round, from Sabadell Asabys venture...
Ortho Awarded BARDA Contract to Accelerate Antigen Test Development and Additional SARS-CoV-2 Antibody Test Milestones
RARITAN, N.J., Sept. 21, 2020 /PRNewswire/ -- Ortho Clinical Diagnostics, a global leader of in vitro diagnostics, today announced its continued collaboration with the Biomedical Advanced Research and Development Authority ( BARDA), part of the Office of the Assistant Secretary for Preparedness an...
Harbour BioMed Announces Two China NMPA Clearances for Clinical Trials for Phase I & Combination Therapy of Next Generation Anti-CTLA-4 Antibody for Treatment of Solid Tumors
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, Sept. 21, 2020 /PRNewswire/ -- Harbour BioMed (HBM), a global, clinical-stage, innovative biopharmaceutical company announced approval of two Investigational New Drug (IND) applications by the China National Medical Products Administr...
Hyde Engineering + Consulting Announces New VP of Global Engineering
BOULDER, Colorado, Sept. 21, 2020 /PRNewswire/ -- Hyde Engineering + Consulting, Inc. (Hyde) announced thatSanjay Shah has accepted the position of Vice President of Global Engineering. Sanjay, based out of Hyde's office in Ahmedabad,India, will provide subject matter expertise in conceptual an...
Key Milestone: The first patient successfully dosed with the candidate drug SR419 for peripheral neuropathic pain
SHANGHAI, Sept. 21, 2020 /PRNewswire/ -- Shanghai SIMR Biotechnology Co., Ltd. (hereinafter referred to as "SIMR" or "Company") announced that, the clinical trial in patients with peripheral neuropathic pain has been initiated for the candidate drug SR419, and the first patient has been successfu...
Viva Biotech and SYNthesis Enters a Strategic Acquisition Agreement, Accelerating the Development of Global Innovative Drug R & D Service Capabilities
SHANGHAI, Sept. 21, 2020 /PRNewswire/ -- Viva Biotech (Shanghai) Co., Ltd. (hereinafter referred to as "Viva Biotech" or "The Company") entered into the Share Purchase Agreement pursuant to which Viva Biotech agreed to acquire and the Vendors agreed to collectively sell 100% of the equity interes...
Innovent and Lilly Jointly Release Phase 3 Results of TYVYT® (Sintilimab Injection) in Combination with GEMZAR® (Gemcitabine) and Platinum Chemotherapy as First-Line Treatment for Squamous Non-Small Cell Lung Cancer at ESMO Virtual Congress 2020
SAN FRANCISCO and SUZHOU, China, Sept. 21, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune and other majo...
Innovent and Lilly Release Biomarker Results of TYVYT® (Sintilimab Injection) in Combination with ALIMTA® (Pemetrexed) and Platinum Chemotherapy as First-Line Treatment for Nonsquamous Non-Small Cell Lung Cancer at ESMO Virtual Congress 2020
SAN FRANCISCO and SUZHOU, China, Sept. 21, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune and other majo...
BAVENCIO Pivotal Phase III JAVELIN Bladder 100 Results Published in The New England Journal of Medicine
Not intended for US-, Canada- and UK-based media DARMSTADT, Germany, and NEW YORK, Sept. 18, 2020 /PRNewswire/ -- Merck and Pfizer Inc. (NYSE: PFE) today announced the publication of detailed results from the Phase III JAVELIN Bladder 100 study online ahead of print inThe New England Journal of...
PolyU designs new dual-task exercise for stroke patients to effectively reduce the risk of falls and fall-related injuries
HONG KONG, Sept. 18, 2020 /PRNewswire/ -- Stroke is the fourth leading cause of death inHong Kong[1], hospitalising around 25,000 people each year[2]. Despite declines in stroke mortality over the past years, it remains one of the major causes of adult disability, greatly affecting the daily live...
First patient in treatment in RhoVac's clinical phase IIb study in Sweden
STOCKHOLM, Sept. 17, 2020 /PRNewswire/ -- RhoVac AB ("RhoVac") announces today, onSeptember 17 2020, that the first patient in Sweden is enrolled in the company's clinical phase IIb study in prostate cancer, a study named RhoVac-002 ("BRaVac"). The first patient in Sweden is enrolled in the cli...
Viva Biotech's Portfolio Company Dogma Therapeutics Reached the Acquisition Agreement with AstraZeneca on Dogma's Oral PCSK9 Inhibitor Program
CAMBRIDGE, Mass., Sept. 17, 2020 /PRNewswire/ -- Dogma Therapeutics ("Dogma"), a Viva Biotech Portfolio Company, has reached an agreement for the acquisition of its oral PCSK9 program by AstraZeneca. Dogma will receive upfront as well as downstream payments linked to global regulatory and commerc...
Global Cord Blood Corporation to Hold Annual General Meeting in Hong Kong on December 7, 2020
HONG KONG, Sept. 17, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) ("GCBC" or the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing, and stem cell storage services, today announced that the Company will hold its 20...
#KIDsforSDGs By Family Mask Academy
HONG KONG, Sept. 17, 2020 /PRNewswire/ -- Family Mask joined forces with Jumpstart Media to organize Jumpstart Kids: Young SDG Leaders (Junior Edition) Program to let the young leaders better understand the role of the United Nations (UN) and the 17 Sustainable Development Goals (SDGs). Young SDG...
Acellular-Convalescent Plasma (A-CP) and New Technologies Introduced by Regen Lab®
LAUSANNE, Switzerland, Sept. 17, 2020 /PRNewswire/ -- Convalescent Plasma (CP), also calledPassive Antibody Therapy, for the treatment of COVID-19 infected patients was approved by the US-FDA two weeks ago. This great news was based on a nationwide evaluation in 14 U.S. medical institutions, and...
Alter Pharma Group Adopts Movilitas.Cloud to Ensure Safer Goods and EU FMD Compliance Throughout the Product Path and Changes in Packaging
Solution supports requirements for traceability down to the single package level and optimizes the repackaging process for increased efficiency and safer medicine COLUMBIA, Maryland, Sept. 16, 2020 /PRNewswire/ -- Movilitas announced that the Alter Pharma Group, a pharmaceutical group active in ...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00