Medical/Pharmaceuticals
Ortho Announces Plans to Accelerate COVID-19 Antigen and Antibody Test Development Through New Contract with BARDA and the Department of Defense
RARITAN, N.J., April 1, 2021 /PRNewswire/ -- Ortho Clinical Diagnostics (Nasdaq: OCDX), one of the world's largest pure-play IVD companies, today announced that through its continued partnership with the Biomedical Advanced Research and Development Authority (BARDA) and the U.S. Department of Def...
Increased Virtual Learning Impacts Children's Vision
Expert panel on vision and health highlights the effects of screen-based learning on children's vision one year into the pandemic DALLAS, April 1, 2021 /PRNewswire/ -- The Vision Impact Institute recently convened experts in children's vision and health todiscuss the implications of digital lear...
Iconovo signs agreement with ISR for the development of inhaled covid-19 vaccine
STOCKHOLM, April 1, 2021 /PRNewswire/ -- Iconovo AB (publ), a company developing complete inhalation products for a global market, today announced that it has signed an agreement with the immunotherapy company Immune System Regulation AB (ISR), an affiliate to ISR Immune System Regulation Holding...
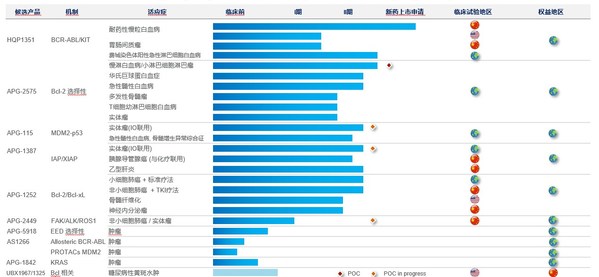
Ascentage Pharma Announces 2020 Annual Results and Reports Launch Readiness for Its Core Drug Candidate
SUZHOU, China and ROCKVILLE, Md., March 31, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today released its annual results for full ...
Five New 'Key Opinion Leaders' Appointed to Blinkcns Scientific Advisory Board
Dr. Angelo Antonini, Dr. Matteo Bologna, Roy Jones, Dr. Fidias Leon-Sarmiento, and Dr.Fabrizio Stocchi, will help guide company as they advance central nervous system research CHARLESTON, South Carolina, March 31, 2021 /PRNewswire/ -- Blinkcns, Inc, a biotechnology company pioneering blink refle...
BIOVAXYS files FDA pre-IND meeting request and briefing package for COVID-T
VANCOUVER, BC, March 31, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA:5LB) (OTC:LMNGF) ("BioVaxys"), the world leader in haptenized antigen vaccines for antiviral and cancer applications, announced today that it is has filed a pre-IND (Investigational New Drug) meeting request ...
Future Health: Innovative Health Around the World
LONDON, March 31, 2021 /PRNewswire/ -- It started with the UK but the renowned organisers of Future Health Innovation have recently announced the launch of a full series of digital events - the Health Week Series. Concentrating on key markets around the world, there will be a Summer and Autumn ev...
CStone Announces China NMPA New Drug Approval of Precision Therapy AYVAKIT® (avapritinib) for the Treatment of Adults with Unresectable or Metastatic PDGFRA Exon 18 Mutant Gastrointestinal Stromal Tumor
SUZHOU, China, March 31, 2021 /PRNewswire/ -- CStone Pharmaceuticals (CStone, HKEX: 2616), a leading biopharmaceutical company focused on developing and commercializing innovative immuno-oncology therapies and precision medicines, today announces that the National Medical Products Administration ...
Evive Biotech Submits Biologics License Application to US FDA for Ryzneuta(TM)
* BLA submission to the U.S. FDA follows successful conclusion of Evive's Global Phase III Clinical Trial for chemotherapy-induced neutropenia in breast cancer patients. * Evive is the first Chinese biologics company to advance a novel biologic product from pre-clinical studies to a complet...
Cigna Study Shows Uncertainty about the Future as Leading Cause of Stress
HONG KONG, March 31, 2021 /PRNewswire/ -- Cigna Hong Kong today released the Hong Kong findings of the latest edition of its Cigna COVID-19 Global Impact Study. As part of Cigna's annual 360 Well-Being Survey, this research is the fourth in a series of studies to better understand the impact of t...
Everest Medicines Announces China NMPA Approval of Clinical Trial Application to Evaluate Trodelvy® in a Phase 2 Basket Trial for a Variety of Cancers with High TROP-2 Expression
SHANGHAI, March 31, 2021 /PRNewswire/ -- Everest Medicines
Menarini Silicon Biosystems launches CELLSEARCH® Circulating Multiple Myeloma Cells Assay, a new non-invasive test to monitor disease status in clinical research studies
BOLOGNA, Italy and HUNTINGDON VALLEY, Pa., March 31, 2021 /PRNewswire/ -- Menarini Silicon Biosystems, a pioneer of liquid biopsy and single cell technologies, announced today the launch of a new assay to meet the needs of pharma companies, CROs, clinicians and research scientists working on mult...
Innovent Announces Parsaclisib (IBI376) was Granted Breakthrough Therapy Designation by the NMPA for the Treatment of Relapsed/Refractory Follicular Lymphoma
SAN FRANCISCO and SUZHOU, China, March 31, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major disea...
Viva Biotech Announced 2020 Annual Results
The Group's Revenue Surged by 115.7% YoY Revenue from the CFS Business Increased Significantly by 146.3% YoY Establish One-stop Service Platform for Novel Drug Discovery and Production via Internal Growth and External Expansion Financial Highlights of the year ended December 31, 2020: * Reve...
binx health Receives FDA CLIA Waiver for Chlamydia and Gonorrhea Test, Expanding Critical Access to Single-Visit Diagnoses
First ever 30-minute, CLIA-waived, molecular PCR Test for CT/NG now available for OBGYN, physician offices and retail settings holding certificates of waiver BOSTON, March 31, 2021 /PRNewswire/ -- binx health, a population health technology company that provides convenient healthcare solutions, a...
European Commission Approves mAbxience's Bevacizumab For The Treatment Of Certain Types Of Cancer
mAbxience, a global fully-fledged biotech company, with over a decade of experience in the development, manufacture and commercialization of biopharmaceuticals, is expanding patient access to biological treatments throughoutEurope. MADRID, March 31, 2021 /PRNewswire/ -- On 31 March 2021, the Eur...
Ajinomoto Bio-Pharma Services Expands Manufacturing Agreement with AstraZeneca to Include Drug Product Manufacturing
WETTEREN, Belgium, March 31, 2021 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce a manufacturing agreement expansion with AstraZeneca to include drug product manufact...
WuXi AppTec Reports Strong 2020 Annual Results
Revenue Up 28.5% Year-over-Year to RMB16,535 Million Net Profit Attributable to Owners of the Company Up 59.6% Year-over-Year to RMB2,960 Million Diluted EPS Up 56.3% Year-over-Year to RMB1.25 Adjusted Non-IFRS Net Profit Attributable to Owners of the Company Up 48.1% Year-over-Year toRMB3,565 Mil...
Innovent Announced Financial Results for Full Year Ended December 31, 2020 and Corporate Progress
SAN FRANCISCO and SUZHOU, China, March 30, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major disea...
Fosun International Announces 2020 Annual Results: Total Revenue Amounted to RMB136.6 Billion, Gathering Strength to Fuel Growth
2020 Annual Results Summary and Recent Highlights: * Total revenue amounted to RMB136.6 billion. Net profit attributable to owners of the parent wasRMB8.02 billion. Industrial operating profit reached RMB8.15 billion. * Global collaboration has been conducted to develop COVID-19 mRNA va...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 280 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 260 media titles]
2026-02-17 14:16