Pharmaceuticals
TraceLink's Magnum Release of its Opus Platform Will Revolutionize Supply Chain Management by Enabling All Businesses to Digitalize their End-to-End Supply Networks
With real-time information from all supply chain partners, Supply Chain Managers can reduce inventory and stock-out while improving service levels and lead times BOSTON, Sept. 4, 2024 /PRNewswire/ -- TraceLink, the largest end-to-end digital network platform for intelligent orchestration of the ...
LakeShore Biopharma Announces Leadership Transitions
GAITHERSBURG, Md., Sept. 4, 2024 /PRNewswire/ -- LakeShore Biopharma Co., Ltd. (Nasdaq: LSB) ("LakeShore Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for ...
Innovent Receives Fast Track Designation from the U.S. FDA for IBI363 (PD-1/IL-2α Bispecific Antibody Fusion Protein) as Monotherapy for Advanced Melanoma
SAN FRANCISCO and SUZHOU, China, Sept. 4, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune...
IPAX-1 Study of TLX101 Investigational Glioblastoma Therapy Published in Neuro-Oncology Advances
MELBOURNE, Australia, Sept. 4, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that the Company's IPAX-1 Phase I study has been published inNeuro-Oncology Advances, confirming the safety and tolerability profile, and early efficacy of TLX101 thera...
Seegene and Springer Nature Launch 'Nature Awards MDx Impact Grants' to Innovate Syndromic PCR Diagnostic Assays
- Nature Awards MDx Impact Grants in partnership with Seegene extends commitment to external innovation. - Global research teams invited to submit proposals to develop new tests for human infectious diseases. Application deadline:December 2, 2024. - Nature Awards to lead the application and eva...
Frost & Sullivan Released Report: "BiBo Pharma: A Global CDMO Leading in Advanced Biopharmaceutical Manufacturing Technologies"
SHANGHAI, Sept. 2, 2024 /PRNewswire/ -- Frost & Sullivan released a report, titled "BiBo Pharma: A Global CDMO Leading in Advanced Biopharmaceutical Manufacturing Technologies". The report explores a global CDMO leading the "The Fourth Revolutionary Wave of Biologics Manufacturing" and pioneering...
Innovent Annouces Multiple Clinical Study Results of Mazdutide to be Presented at the EASD 2024
SAN FRANCISCO and SUZHOU, China, Sept. 2, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic...
Pierre Fabre Laboratories receives European Commission Approval for BRAFTOVI®(encorafenib) in combination with MEKTOVI® (binimetinib) for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAFV600E mutation
* European approval is based on results from the Phase II PHAROS trial, which showed a meaningful clinical benefit to BRAFV600E mutated advanced NSCLC patients with an objective response rate (ORR) of 75% in treatment-naïve patients and 46% in previously treated patients.[1-3] The safety profil...
Guardant Health Japan receives regulatory approval of Guardant360® CDx liquid biopsy as companion diagnostic for amivantamab-vmjw to identify patients with inoperable or recurrent NSCLC harbouring EGFR exon 20 insertion mutations
* Guardant360® CDx is the first blood-based companion diagnostic to be approved inJapan for the detection of EGFR exon 20 insertion mutations TOKYO, Aug. 30, 2024 /PRNewswire/ -- Guardant Health Japan Corp. (HQ: Minato-ku, Tokyo / Representative Director:Mika Takaki), a leading precision oncolog...
Viva Biotech Announces Its 2024 Interim Results: Significant Improvement in Net Profit Growth and Ongoing Optimization of Emerging Technology Platforms
Results Highlights for Interim Results ended 30 June 2024 Revenue reached RMB981.8 million Gross profit amounted to RMB339.1 million Net profit grew by 956.0 YoY to RMB144.2 million Adjusted non-IFRS net profit reached RMB168.2 million, increased by 15.1% YoY HONG KONG, Aug. 29, 2024 /PRNewswire/ ...
LOTTE Holdings establishes a new Healthcare and Biopharmaceutical Corporate Venture Capital
TOKYO, Aug. 29, 2024 /PRNewswire/ -- LOTTE Holdings Co., Ltd. ("LOTTE
Holdings") is pleased to announce the establishment of a new Healthcare and
Biopharmaceutical Corporate Venture Capital ("CVC") dedicated to investing in
biopharmaceuticals and next-generation modalities.
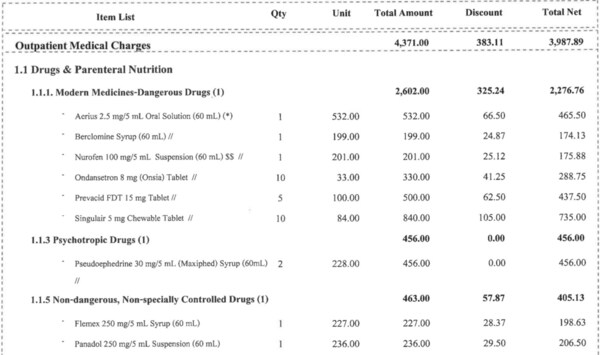
Necessary Care or Abuse? Unpacking the Reason Behind Overprescription
SINGAPORE, Aug. 29, 2024 /PRNewswire/ -- Have you ever wondered if the ten prescriptions your doctor gave you for a cold are truly necessary? Or is there a more complex issue of abuse happening behind the scenes? Today's Case: Overcharging a 7-Year-Old Somsak (an alias name), a 7-year-old boy fr...
Innovent to Present Clinical Data of Multiple Novel Molecules at WCLC and ESMO 2024
SAN FRANCISCO and SUZHOU, China, Aug. 29, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune...
Innovent Announces 2024 Interim Results and Business Updates
Strong commercial performance and significant pipeline milestones support sustained growth and innovation SAN FRANSISCO and SUZHOU, China, Aug. 28, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and com...
Everest Medicines Announces Interim Results for First Half of 2024
SHANGHAI, Aug. 28, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the development, manufacturing and commercialization of innovative medicines and vaccines, today announced its interim results for the first half of 2024 a...
Telix Submits NDA for TLX101-CDx (Pixclara®) Brain Cancer Imaging Agent
MELBOURNE, Australia, Aug. 28, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces it has submitted a New Drug Application (NDA) tothe United States (U.S.) Food and Drug Administration (FDA) for TLX101-CDx, (Pixclara®[1], 18F-floretyrosine or 18F-FET)...
Unique International Collaboration Brings Breakthrough Oral Cholera Vaccine to Market
Singapore-based Hilleman Laboratories, along with Bharat Biotech, confirm launch of new cholera vaccine, HILLCHOL® Success provides "template for the internationalization of future vaccine and biologics development fromSingapore" – Dr. Raman Rao, CEO, Hilleman Laboratories Hilleman Laboratories ...
Boan Biotech Announces 2024 Half Year Results: Revenue Grows by 39%, Net Profit Increases by RMB 180 Million
YANTAI, China, Aug. 27, 2024 /PRNewswire/ -- Boan Biotech (6955.HK) today announced its 2024 half-year results and latest developments. During the reporting period, the company's total revenue was RMB 363 million, up 39% year-over-year. In this, the revenue from product sales wasRMB 332 million,...
Currax Pharmaceuticals: CONTRAVE®/MYSIMBA® Demonstrates Positive Cardiovascular Safety in a Large, Real-World Evidence Study
BRENTWOOD, Tenn., Aug. 27, 2024 /PRNewswire/ -- Currax Pharmaceuticals LLC ("Currax") today announced the results of a Cardiovascular Health Outcomes Analysis (HOA). The results showed there is no evidence of excess cardiovascular risk and no statistically significant difference in major adverse...
JW Therapeutics Announces NMPA Approval of the Supplemental Biological License Application for Carteyva® in Adult Patients with Relapsed or Refractory Mantle Cell Lymphoma
SHANGHAI, Aug. 27, 2024 /PRNewswire/ -- JW Therapeutics (HKEx: 2126), an independent and innovative biotechnology company focusing on developing, manufacturing and commercializing cell immunotherapy products, announced that the National Medical Products Administration (NMPA) ofChina has approv...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 318 media titles]
2026-01-29 15:06Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 309 media titles]
2026-01-27 13:00Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 294 media titles]
2026-01-27 18:00Multi-Award Winning Phuket Marriott Resort & Spa, Merlin Beach: Voted Top 3 Best Family Resort
[Picked up by 283 media titles]
2026-01-30 08:00True 1000Hz Powerhouse: PHILIPS EVNIA Unleashes the World's First 1000Hz Dual-Mode Gaming Monitor 27M2N5500XD
[Picked up by 282 media titles]
2026-01-29 18:03