Biotechnology
PharmAbcine to Present at the BIO Digital International Convention 2021
DAEJEON, South Korea, June 2, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of antibody therapeutics, announced today that the company will participate in BIO Digital International Convention. The company will participate in...
Samsung Biologics and KAHR Medical Announce Development and Manufacturing Agreement for Cancer Immunotherapy Drug Candidate
INCHEON, South Korea, June 2, 2021 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), the world's leading contract development and manufacturing organization, signed a strategic partnership with KAHR Medical Ltd., a cancer immunotherapy company developing novel multifunctional immune-recruitment...
ImmVira will present the U.S. Clinical Phase I Study Results of MVR-T3011 via Intratumoral Administration at ASCO 2021
SHENZHEN, China, June 2, 2021 /PRNewswire/ -- ImmVira will present results from
its clinical Phase I study of MVR-T3011 via intratumoral (IT) administration at
the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4-
8, 2021 for the first time.
Bridge Biotherapeutics Announces the Initiation of the Proof of Mechanism Study for BBT-401 in Patients with Ulcerative Colitis; Rectal Delivery of Drug to Target Site
* The new proof of mechanism trial in ulcerative colitis had its first patient dosed inNew Zealand * In parallel with the oral-administered, proof of clinical principle study, the new proof of mechanism study will examine the efficacy of BBT-401 when delivered directly to the target organ via...
Eucure Biopharma to Present Findings From Anti-CD40 and Anti-CTLA-4 mAb Clinical Trials at the 2021 ASCO Meeting
BEIJING and BOSTON, June 1, 2021 /PRNewswire/ -- Eucure Biopharma will present findings from two Phase I clinical trials at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, held fromJune 4th to 8th. The first trial (poster #2580; NCT04481009) is designed to assess the safety...
GenScript ProBio and Neoletix signed a letter of intent on clinical and commercial production of recombinant human coagulation factor VIII
NANJING, China, June 1, 2021 /PRNewswire/ -- On June 1st 2021, GenScript ProBio and Neoletix (Beijing) Biotechnology Co., Ltd. (hereinafter referred to as Neoletix) signed a letter of intent on cooperation for clinical and commercial production of human coagulation factor VIII. GenScript ProBio s...
Neurophth Therapeutics and Hopstem Biotechnology Announce Strategic Partnership to Develop Human Induced Pluripotent Stem Cell-Derived Therapies for the Treatment of Ocular Diseases
HOUSTON and SAN DIEGO, June 1, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd., a fully-integrated genetic medicines company developing AAV-mediated gene therapies for the treatment of ocular diseases, and Hopstem Biotechnology, the leading human induced pluripotent stem cell (hiPSC) and neural...
Samsung Biologics to add mRNA vaccine drug substance manufacturing suite to expand portfolio of services
* Samsung Biologics, a global leading CDMO, will add mRNA vaccine DS production capability to its existing facility by the first half of 2022 * The addition will enable Samsung Biologics to provide end-to-end mRNA vaccine manufacturing services from bulk drug substance to aseptic fill/finish ...
Samsung Biologics Adds Four Global ISO Certifications For BCMS, Energy, Health & Safety, and Environmental Management
* Expanding upon its existing ISO22301 certification, Samsung Biologics is now fully certified with Business Continuity Management System for Security and Resilience across all business areas * Samsung Biologics adds ISO50001, ISO45001, ISO14001 for Energy Management, Occupational Health and ...
AffaMed Therapeutics Announces Approval for the Phase IIb Clinical Study of AM006 in China for the Treatment of Parkinson's Disease
SHANGHAI, May 27, 2021 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical stage biopharmaceutical company dedicated to addressing critical unmet medical needs in ophthalmic, neurological and psychiatric disorders, today announced thatChina's National Medical Products Administrati...
Menarini Silicon Biosystems launches new breakthrough DEPArray™ PLUS image-based cell sorter to isolate rare cells with single cell precision
DEPArray™ PLUS – the new automated and versatile platform to investigate pre-selected rare cell populations from various samples with 100% purity, now enhanced with 9 fluorescent channels and remarkably improved user experience BOLOGNA, Italy and HUNTINGDON VALLEY, Pa., May 27, 2021 /PRNewswire/ ...
TransThera announces collaboration with Roche to evaluate TT-00420 as part of a novel combination to treat GI cancers in China
NANJING, China, May 26, 2021 /PRNewswire/ -- TransThera Biosciences Co. Ltd. ( "TransThera" ), a clinical stage biotechnology company dedicated to developing innovative therapeutics across oncology, cardiovascular, and inflammatory diseases with major unmet medical needs globally and inChina, to...
Gracell Biotechnologies Hosting Key Opinion Leader Webinar on High Risk Multiple Myeloma, Treatment Challenges and Dual-Targeting CAR-T with Overnight Manufacturing - a Novel Approach for Treatment of Multiple Myeloma
SUZHOU, China and PALO ALTO, Calif., May 26, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to discovering and developing highly efficacious and affordable cell therapies for the treatment of cancer, today ...
Prosit Sole Biotechnology Initiates First-In-Human Clinical Trial of Novel Interferon Lambda Chimera in US
BALTIMORE, May 26, 2021 /PRNewswire/ -- Prosit Sole Biotechnology, a clinical-stage biotech company developing novel protein therapeutics, today announces that it has initiated the first-in-human ("FIH") Phase I clinical trial of PSP001 in US. PSP001 is a novel, long acting and potent interferon ...
I Peace's cell manufacturing facility "Peace Engine Kyoto" receives third-party certification as US cGMP compliant: the facility now meets the standard for both the US and Japanese markets
PALO ALTO, Calif., May 25, 2021 /PRNewswire/ -- I Peace, Inc. (CEO: Koji Tanabe ), aPalo Alto-based biotech start-up focusing on Nobel Prize-winning technology "induced pluripotent stem cells (iPSCs)" received a third-party certification as US FDA cGMP compliant for its manufacturing facility "Pea...
Procyrion Announces Successful First-in-Human Cases in Cardiorenal Syndrome (CRS) Patients with Aortix™ Percutaneous Mechanical Circulatory Support Device
Pilot CRS Study to Evaluate Performance of Novel Aortix device in Australia and the U.S. HOUSTON, May 25, 2021 /PRNewswire/ -- Procyrion, Inc., a medical device company dedicated to improving outcomes for patients with cardiac and renal impairment, announced today successful treatment of the fir...
Singapore-based HeMo Bioengineering Receives China's NMPA Approval for flagship Afentta(TM) Aspiration Catheter
SINGAPORE, May 24, 2021 /PRNewswire/ -- HeMo Bioengineering Ltd ("HeMo"), a Singapore-based medical device company with a focus on treating stroke patients, is pleased to announce that its Afentta™ intracranial thrombectomy aspiration catheter – a product developed byHeMo's branch in China – has ...
A 'Game Changer' COVID-19 drug from Korea was introduced in a world class academic journal
SEOUL South Korea, May 23, 2021 /PRNewswire/ -- Hyundai Bioscience (KOSDAQ 048410) announced on the 24th that CNPharm, its major shareholding bio tech company, published an article containing the research results of CP-COV03, a Niclosamide-based oral treatment for COVID-19 based on its proprietar...
Moderna and Samsung Biologics Announce Agreement for Fill-Finish Manufacturing of Moderna's COVID-19 Vaccine
CAMBRIDGE, Mass. and INCHEON, South Korea, May 22, 2021 /PRNewswire/ -- Moderna (Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, and Samsung Biologics (KRX: 207940.KS), a leading global CDMO providing a fully integrated end-to-end contract develop...
Kintor Announced (1) FDA Has Greenlighted Proxalutamide's Phase III Study for Hospitalized Male and Female COVID-19 Patients to Be Conducted; and (2) Inclusion of Female Outpatients in Proxalutamide's Phase III Study for Mild to Moderate COVID-19
SUZHOU, China, May 19, 2021 /PRNewswire/ -- On May 18, Kintor Pharmaceutical Limited (HKEX.9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, announced that the U.S. Food and Drug Administration (FDA) has greenlighted proxalutamide's ph...
Week's Top Stories
Most Reposted
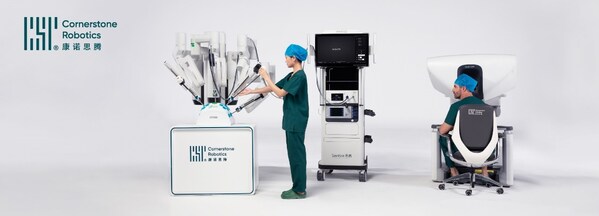
Cornerstone Robotics Raises over US$70 million Funding to Forge Accessibility in Robotic Surgery
[Picked up by 321 media titles]
2025-01-13 09:05URBAN REVIVO Celebrates Lunar New Year with Unique Collaboration with Artist Mercedes Bellido
[Picked up by 293 media titles]
2025-01-13 10:44Gen Z Travelers Drive Buy Now, Plan Later Travel Bookings by Over 20%: Fliggy's 2024 Buy Now Plan Later Travel Insight
[Picked up by 286 media titles]
2025-01-10 16:04VANTAGE FOUNDATION SUPPORTS GRAB INDONESIA IN EMPOWERING WOMEN DRIVER-PARTNER
[Picked up by 280 media titles]
2025-01-09 18:26LANDI Global Unveils Flagship Cx20: Elevating business efficiency and customer experience with a next-generation Windows-powered terminal
[Picked up by 274 media titles]
2025-01-11 22:30