Biotechnology
Samsung Biologics listed among top most sustainable companies in Dow Jones Sustainability World Index
* Acknowledged for strong environmental, social, and governance (ESG) performance and continued commitment to sustainability through net-zero initiatives and responsible value chain management INCHEON, South Korea, Dec. 16, 2024 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), a global contr...
Microbio Co., Ltd. (4128) Announces Promising Results of "MS-20" in Combination with Keytruda for Stage IIIb/IV Non-Small Cell Lung Cancer
CUPERTINO, Calif., Dec. 16, 2024 /PRNewswire/ -- Microbio Co., Ltd. (4128) today revealed encouraging results from an exploratory clinical trial of MS-20 combined with Keytruda (pembrolizumab) in stage IIIb/IV non-small cell lung cancer (NSCLC). The trial showed a threefold increase in the object...
Nona Biosciences Announces Discovery Collaboration with Candid Therapeutics for Next-Generation T-Cell Engagers
CAMBRIDGE, Mass., Dec. 16, 2024 /PRNewswire/ -- Nona Biosciences, a global biotechnology company providing a total solution from "Idea to IND" (I to ITM ), today announced a research collaboration and license agreement to discover next-generation T-cell engagers ("TCEs") with Candid Therapeutics, ...
Young International Scientists Say: China-Myanmar Partnership Advances Agricultural Innovation
BEIJING, Dec. 13, 2024 /PRNewswire/ -- Mango, a tropical fruit rich in nutrients, has a vital position in Myanmar's agriculture.Myanmar researcher Nann Miky Moh Moh worked with Chinese scientists at Zhejiang University in east China on genetically improving the flavor and variety of the fruit. ...
Accropeutics Inc. Announces U.S. FDA Clearance of IND Application for RIPK2 Inhibitor AC-101
* Company to conduct a Phase II multi-regional clinical trial (MRCT) for the treatment of Ulcerative Colitis (UC) * A Phase Ib Study of AC-101 for Ulcerative Colitis (UC) has been initiated in China * AC-101 demonstrated a favorable safety and PK/PD profile in healthy volunteers in Phase Ia ...
Transcenta Debuts Promising Anti-Tumor Activity of Novel LIV-1 Targeting ADCs with Site-Specific Conjugated Topo I Inhibitor Payload in TNBC Tumor Models at 2024 SABCS
PRINCETON, N.J. and SUZHOU, China, Dec. 12, 2024 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a global clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, ann...
Bota Bio debuted the 2024 FiE and launched its subsidiary HeliaGenesis, focusing on food and nutrition industries
SAN FRANCISCO and HANGZHOU, China, Dec. 12, 2024 /PRNewswire/ -- The 31st Food Ingredients Europe (referred to as FiE) concluded successfully fromNovember 19th to 21st, 2024, in Frankfurt, Germany. The event brought together over 1,500 ingredient manufacturers and more than 25,000 professional bu...
CureGene Received Clinical Trial Approval in China for CG-0255 for Injection - A Novel Antiplatelet Therapy
SHANGHAI, Dec. 12, 2024 /PRNewswire/ -- CureGene Pharmaceutical ("CureGene"), a biotechnology company dedicated to innovative treatments for critical unmet medical needs in cardio-cerebrovascular and antiviral disease, proudly announces the approval of its Clinical Trial Application by theChina's...
Porton Pharma Solutions and Aojin Life Sciences Announce Strategic Collaboration to Advance XDC Conjugate Drugs for Dual IND Filings in China and the U.S.
CHONGQING, China, Dec. 12, 2024 /PRNewswire/ -- Recently, Porton Pharma
Solutions Ltd. ("Porton") announced a strategic partnership with Aojin Life
Sciences (Yixing) Co., Ltd. ("Aojin Life Sciences").
SK bioscience Receives Approval for Global Clinical Trials of mRNA Japanese Encephalitis Vaccine Candidate
* SK bioscience's first mRNA vaccine candidate is expected to enter clinical trials inAustralia in February 2025, with interim results expected by 2026. * Joint R&D project between SK and CEPI includes up to USD 40 million in initial funding support from CEPI. * SK bioscience will respond to...
WuXi Biologics Included in UNGC 20 Case Examples of Sustainable Development for 20 Years Collection
* Leading Green CRDMO Driven by Innovation for a Healthier Future * Offering End-to-end Green Biologics Solutions for the Entire Industry SHANGHAI, Dec. 12, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development, and Manufacturing Organizati...
KIND Presents Preclinical Results of AND017 in Animal Models of Sickle Cell Disease (SCD), Myelodysplastic Syndromes (MDS), and β-Thalassemia at the 66th American Society of Hematology Meeting and Exposition and Announces FDA Authorization of Investigational New Drug (IND) Application for AND017 for Clinical Trial in Sickle Cell Disease (SCD)
SAN FRANCISCO, Dec. 12, 2024 /PRNewswire/ -- Kind Pharmaceutical ("Hangzhou Andao Pharmaceutical Ltd. and Kind Pharmaceuticals LLC"), a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, today announced that preclinical...
Laekna Announces Poster Presentation on Internally Discovered Selective PI3Kα Inhibitor at SABCS 2024
LOS ANGELES, Dec. 11, 2024 /PRNewswire/ -- Laekna (2105.HK) today announced that the company will present an internally discovered preclinical candidate at the 2024 San Antonio Breast Cancer Symposium (SABCS) onDecember 13, 2024. The presentation will feature preclinical characterization of LAE11...
Nature Biotechnology | Generative Chemistry Enables Insilico to Develop Gut-Restricted PHD Inhibitors Promising for Intestinal Mucosal Barrier Repair and Immunomodulation
* The study presents the journey of designing and optimizing novel gut-restricted PHD inhibitors using the comprehensive generative chemistry engine Chemistry42. * From initiation to preclinical candidate nomination, the program spanned only 12 months, during which approximately 115 molecules...
Dizal Announces Positive Pooled Data of Sunvozertinib in EGFR Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer Published in Lung Cancer
Sunvozertinib, as a single agent, demonstrated promising antitumor activity and favorable safety profile in heavily pretreated patients with EGFR-mutated NSCLC who had developed resistance to EGFR TKI treatment SHANGHAI, Dec. 11, 2024 /PRNewswire/ -- Dizal (SSE:688192), a biopharmaceutical compa...
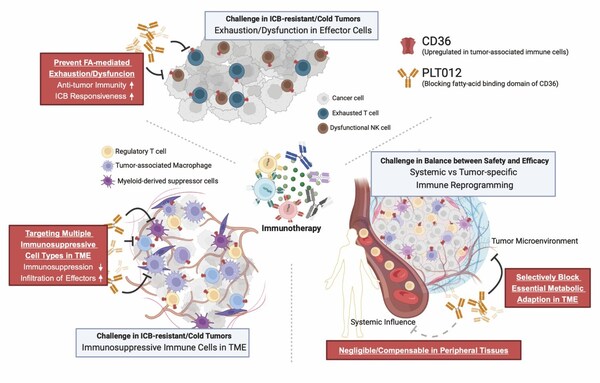
Pilatus Biosciences Inc. Secures FDA Orphan Drug Designation for PLT012: A Breakthrough in Liver and Intrahepatic Bile Duct Cancer Treatment
DOVER, Del. and EPALINGES, Switzerland, Dec. 10, 2024 /PRNewswire/ -- Pilatus Biosciences Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to its leading molecule, PLT012, for treating liver and intrahepatic bile duct cancer (HCC/ICCA). Ach...
【2024 ASH】TransThera Announces the Phase I Study Results of TT-01488, a Novel Non-Covalent BTK Inhibitor, in Patients with Relapsed or Refractory B-cell Malignancies
NANJING, China and GAITHERSBURG, Md., Dec. 10, 2024 /PRNewswire/ -- TransThera Sciences (Nanjing), Inc. (the "TransThera"), a clinical demand-oriented, registrational clinical stage biopharmaceutical company focusing on discovering and developing innovative small molecule therapies for oncology, ...
PENTAX Medical obtained US FDA 510(k) clearance for new models of the PENTAX Medical i20c Video Endoscope Series
MONTVALE, N.J., Dec. 10, 2024 /PRNewswire/ -- PENTAX Medical, a division of HOYA Group, has obtained US FDA 510(k) clearance for new models of the PENTAX Medical i20c Video Endoscope Series—PENTAX Medical Video Colonoscope EC34-i20cL, PENTAX Medical Video Upper GI Scope EG27‑i20c and Right/Left W...
Nona Biosciences and Kodiak Sciences Partner on Next-Generation Antibody Therapies for Ophthalmic Diseases
CAMBRIDGE, Mass., Dec. 9, 2024 /PRNewswire/ -- Nona Biosciences, a global biotechnology company providing a total solution from "Idea to IND" (I to ITM), ranging from target validation and antibody discovery through preclinical research, today announced a collaboration with Kodiak Sciences Inc. (...
IASO Bio Presented Study Findings on the Impact of CAR T-Cell Persistence on Clinical Outcomes in Relapsed/Refractory Multiple Myeloma with Equecabtagene Autoleucel(FUCASO) Myeloma at 2024 ASH
SHANGHAI and NANJING, China and SAN JOSE, Calif., Dec. 9, 2024 /PRNewswire/ -- IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company focused on the discovery, development, manufacturing, and commercialization of innovative cell therapies and biologics, today shared findings via a poster ...
Week's Top Stories
Most Reposted
AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 315 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 311 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 305 media titles]
2026-02-03 12:00Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 296 media titles]
2026-02-09 10:00AIA RESEARCH REVEALS INGRAINED HEALTH STEREOTYPES ARE HOLDING BACK WELLBEING IN ASIA
[Picked up by 285 media titles]
2026-02-09 16:08