Biotechnology
Bloomage 'YunZhong (In Cloud) Public Welfare' Campaign: A 14-Year Journey of Cultural Preservation and Sustainability
PARSIPPANY, N.J., Oct. 22, 2024 /PRNewswire/ -- Bloomage, a global leader in the research, development, production, and sale of hyaluronic acid and other bioactive substances, continues to showcase its leadership in Environmental, Social, and Governance (ESG) initiatives. The company'sYunZhong (I...
Minghui Pharmaceutical Inc. Announces Positive Topline Results from Phase Ib/II Clinical Trial of MHB018A, a Subcutaneous Single-Domain IGF-1R Antibody, in Patients with Active, Moderate-to-Severe Thyroid Eye Disease (TED)
SHANGHAI, Oct. 22, 2024 /PRNewswire/ -- Minghui Pharmaceutical, Inc., a late-stage biopharmaceutical company dedicated to developing transformative medicines in immunology and oncology, today announced positive topline results from its Phase Ib/II clinical trial of MHB018A in patients with active...
HELP Therapeutics announces FDA clearance of IND application for universal iPSC-derived HiCM-188 cell therapy for the treatment of end-stage heart failure
NANJING, China, Oct. 22, 2024 /PRNewswire/ -- HELP Therapeutics Co. Ltd, a clinical-stage cell therapy company,today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for "Allogeneic Human iPSC-derived cardiomyocytes (HiCM-188) a...
Origin Agritech Establishes Biotechnology Service Consortium to Accelerate Licensing and Commercialization of GMO and Gene Editing Technologies
BEIJING, Oct. 21, 2024 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), a leading Chinese agricultural technology company, is pleased to announce the establishment of the "Origin Marker Biological Breeding Service Consortium" in partnership with China Golden Marker...
NIANCE Unveils Groundbreaking White Paper on Beauty and Longevity: Addressing the 12 Hallmarks of Aging
ZURICH, Oct. 21, 2024 /PRNewswire/ -- NIANCE, the Swiss luxury skincare and wellness leader, announce the release of its new white paper,"Addressing the 12 Hallmarks of Aging." This first-of-its-kind document dives into the evolving science of aging and showcases how NIANCE's innovative products ...
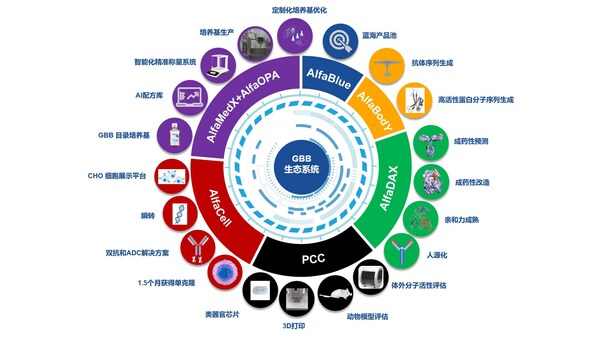
Great Bay Bio Intelligent Drug Development Ecosystem Officially Launched
HONG KONG, Oct. 20, 2024 /PRNewswire/ -- On October 18th, the new product launch event hosted by Great Bay Bio (hereinafter referred to as "GBB") was successfully held in Suzhou, where industry players gathered to celebrate the feast of technological innovation. At the event, GBB announced the of...
MicuRx Pharmaceuticals Showcases the Pipeline Status of MRX-5 and MRX-8 at BIO Investor Forum
SAN FRANCISCO, Oct. 18, 2024 /PRNewswire/ -- Dr. Regis Vilchez, Chief Medical Officer of MicuRx Pharmaceuticals, presented the latest advancements of company's development program, MRX-5 and MRX-8, at the BIO Investor Forum (BIF). These development programs offer new potentials for the future tre...
LaNova Medicines Announces Initiation of Phase 1 Clinical Trial of Anti-PD-1/VEGF Bispecific Antibody LM-299 and Completion of $42 Million Series C1 Financing
* Phase 1 trial of LM-299, an anti-PD-1/VEGF BsAb, initiated in China for advanced solid tumors following promising preclinical results demonstrating strong inhibition of tumor growth and well-tolerated safety profile * IND for LM-299 in the US expected to be submitted in the second half of 20...
111 to Announce Third Quarter 2024 Unaudited Financial Results on November 28, 2024 - Conference Call to Follow
SHANGHAI, Oct. 18, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to reshaping the value chain of healthcare industry by digitally empowering the upstream and downstream inChina, today announced that it will repor...
Exclusive Interview with Lang Guojun, founder of Sanyou Bio: The Key to Breaking the Bottleneck of New Drug Innovation--The Past and Future of the Super-Trillion Molecule Library
SHANGHAI, Oct. 17, 2024 /PRNewswire/ -- In the ninth year since the establishment of Sanyou Bio, Lang Guojun believes that the time is perfect to unveil the world's largest super-trillion molecule library. During the interview with Tongxieyi, he frankly and confidently expressed, "Nine years of ...
Chipscreen Biosciences' Innovative Anti-Tumor Drug CS231295 Tablets: Investigational New Drug (IND) Application Accepted
SHENZHEN, China, Oct. 17, 2024 /PRNewswire/ -- On October 17, 2024, Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) submitted the company's Investigational New Drug (IND) application for CS231295 tablets, a Class 1 innovative drug for the treatment of t...
Amplo Biotechnology Announces Three Presentations at the European Society of Gene and Cell Therapy 31st Annual Congress
SAN DIEGO, Oct. 17, 2024 /PRNewswire/ -- Amplo Biotechnology, a biotechnology company developing genetic medicines for the treatment of neuromuscular diseases, announced the presentation of three posters at the European Society of Gene and Cell Therapy (ESGCT) 31st Annual Congress, taking place f...
KIND Announces Late-Breaking Abstract Accepted for Presentation on AND017 Clinical Trials to Treat Anemia in Chronic Kidney Disease (CKD) at ASN - Kidney Week 2024
SAN FRANCISCO, Oct. 17, 2024 /PRNewswire/ -- Kind Pharmaceutical ("Hangzhou Andao Pharmaceutical Ltd. and Kind Pharmaceuticals LLC"), a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, today announced that the late-br...
WuXi Biologics Receives ESG Corporate Platinum Award from The Asset for Fourth Consecutive Year
HONG KONG, Oct. 16, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that it has received a Platinum Award from The Asset ESG (Environmental, Social and Governance) Corporate Awards ...
IGCS Late Breaking Abstract and The Lancet: Akeso Published Positive PFS and OS Results from Phase 3 First-Line Study of Cadonilimab in Cervical Cancer
HONG KONG, Oct. 16, 2024 /PRNewswire/ -- Akeso Biopharma (9926.HK) (" Akeso", the "Company" ) announced positive results in progression-free survival (PFS) and overall survival (OS) from its Phase 3 clinical study (COMPASSION-16/AK104-303). This study evaluated the efficacy of its independently...
TenNor Announces More than 300 Million RMB Financing to Support Development and Commercialization of Late-Stage Assets Including Rifasutenizol for Heliobacter pylori Infections
SUZHOU, China, Oct. 16, 2024 /PRNewswire/ -- TenNor Therapeutics, a clinical stage company dedicated to developing new therapies to address unmet needs in infectious diseases, announced today the initial closing of a Series E financing round for more than300 million RMB. New investor AMR Action F...
Join the GenScript Biotech Global Forum: Unlocking Innovations in Cell and Gene Therapies
PISCATAWAY, N.J., Oct. 16, 2024 /PRNewswire/ -- The global cell and gene therapy (CGT) industry is poised for remarkable growth, driven by significant advances in life sciences and robust investments from capital markets. To strengthen this vital sector, GenScript is bringing together leading sc...
PharmAust affirms corporate strategy with name change to Neurizon Therapeutics
MELBOURNE, Australia, Oct. 15, 2024 /PRNewswire/ -- Neurizon Therapeutics Limited (ASX: NUZ) ("Neurizon" or "the Company"), a clinical-stage biotech company dedicated to advancing treatments for neurodegenerative diseases, is pleased to announce it has officially changed its name from PharmAust L...
Chiglitazar for the Treatment of MASH Phase II Clinical Study Selected for Oral Presentation at the 2024 American Liver Disease Annual Meeting
SHENZHEN, China, Oct. 15, 2024 /PRNewswire/ -- On October 15, 2024, the list of selected candidates for the oral report of the American Association for the Study of the Liver (AASLD 2024) annual meeting was officially announced online. The abstract of the Phase II clinical study (CGZ203 trial) on...
Neurophet-AriBio to develop 'Next-Gen Platform for Alzheimer's diagnosis'
- Neurophet's brain MRI analysis technology incorporates fluid biomarker data from AriBio's global Phase 3 clinical trial for Alzheimer's disease - Collaborates leveraging Neurophet's medical image analysis technology and AriBio's clinical trial data analysis expertise SEOUL, South Korea, Oct. 1...
Week's Top Stories
Most Reposted
Edufrienz 99: One of Asia's First Digital Platform Advancing Character Education for Future-Ready, Compassionate Children
[Picked up by 318 media titles]
2026-01-23 11:03Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 308 media titles]
2026-01-27 13:00UOB prices AUD2 billion in five-year senior unsecured notes
[Picked up by 306 media titles]
2026-01-23 09:00Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 297 media titles]
2026-01-22 15:16Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 292 media titles]
2026-01-27 18:00