Health Care/Hospital
Crystal Pharmatech Recognized as "Best Partner" by Allorion Therapeutics for Excellent Services
SUZHOU, China, July 7, 2023 /PRNewswire/ -- Crystal Pharmatech, a leading pharmaceutical technology company, has received dual recognition from its esteemed partner, Allorion Therapeutics (Boston, US / Guangzhou, China) who is an innovation-based biotech company aiming at developing novel drug mo...
280 Bio receives IND approval from the FDA for YL-17231
---The Company announces to initiate Phase 1 Clinical Trial with the KRAS inhibitor YL-17231 in US-- SHANGHAI and SOUTH SAN FRANCISCO, Calif. , July 7, 2023 /PRNewswire/ -- 280Bio, Inc. a clinical stage biotechnology company focused on the development of precision oncology medicines, today annou...
Forge Stronger Partnership for Advanced ADC Development! GeneQuantum Healthcare and Aimed Bio collaborate to develop five innovative ADC drugs
SUZHOU, China, July 6, 2023 /PRNewswire/ -- GeneQuantum Healthcare, a leader in the innovative bioconjugation technologies for ADC new drug development, announced the expansion of its partnership with a South Korean biotech company Aimed Bio today. This strategic collaboration aims to jointly dev...
Hexvix®, A Diagnostic Drug for Bladder Cancer of Asieris has Completed the Phase III bridging trial Enrollment
SHANGHAI, July 6, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced the completion of patient enrollment for...
Genesis MedTech Receives Approval for Launch in China for ArtiSential™
SINGAPORE, July 6, 2023 /PRNewswire/ -- Genesis MedTech, a leading medical device company, today announced that its ArtiSential™, a revolutionary series of articulating laparoscopic instruments, has successfully obtained approval from theChina's National Medical Products Administration (NMPA) for...
Registrational Pivotal Phase III Study of Olverembatinib for the First-Line Treatment of Patients with Ph+ ALL Approved by the CDE in China
SUZHOU, China and ROCKVILLE, Md., July 5, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that the Center for Drug Evaluation (CDE) of the Chi...
United Imaging, Kicking off a Birthday Celebration of Five Years in the U.S. Market, Will Debut Three New Scanners at AHRA
Following on the heels of the unveiling of the uMI Panorama™ at SNMMI's annual meeting this week, United Imaging confirmed it will officially debut three additional new products in MRI, CT, and X-ray in July inIndianapolis and enter its second year of the Leaders of Choice program it sponsors wit...
Biosion, Inc. Appoints Furqan Ahmed, PharmD as Vice President and Head of Business Development
NEWARK, Del. and NANJING, China, July 5, 2023 /PRNewswire/ -- Biosion, Inc. ("Biosion"), a global, clinical-stage biotechnology company, today announced the appointment ofFurqan Ahmed, PharmD., as the Vice President and Head of Business Development. In this position, Furqan will be responsible fo...
Lunit Makes Full-Fledged Entry into the Middle East with Participation in 'Saudi Vision 2030' Healthcare Transformation Project
* Lunit's AI-powered cancer screening solutions have been selected for Seha Virtual Hospital's AI evaluation and clinical integration project * Successful validation to be followed by supply contract to assist Saudi Arabia's national cancer and tuberculosis screening program SEOUL, South Korea...
PENTAX MEDICAL ANNOUNCES THE APPOINTMENT OF DAVID HARRISON AS THE NEW PRESIDENT FOR THE AMERICAS
MONTVALE, N.J., July 5, 2023 /PRNewswire/ -- PENTAX Medical, a healthcare industry leader in diagnostic and therapeutic endoscopy, is pleased to announce the appointment ofDavid Harrison as President of the Americas. Effective July 1, 2023, Mr. Harrison assumed the role of President following th...
TiumBio and Hansoh Pharma Announce Clinical Trial Approval of 'HS-10518/TU2670' from NMPA in China
- Hansoh plans to develop 'TU2670 (Hansoh's code: HS-10518)' as a best-in-class novel GnRH antagonist inChina - TiumBio will complete the last patient dosing in a Phase 2a clinical trial of TU2670 in endometriosis inEurope by the end of the year BOSTON and SEONGNAM, South Korea, July 5, 2023 /P...
I-Mab Announces Publication of Claudin18.2 x 4-1BB Bispecific Antibody Givastomig in JITC
GAITHERSBURG, Md. and SHANGHAI, July 5, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of pioneering immunotherapies, today announced the publication of a manuscript entitled "C...
BGI CEO Yin Ye Advocates for Global Technology Sharing
SHENZHEN, China, July 5, 2023 /PRNewswire/ -- Speaking on a panel at the 10th Arab-China Business Conference inRiyadh, Saudi Arabia, Dr Yin Ye, BGI Group CEO, reinforced the importance of making technological advancements available to everyone through scientific sharing to benefit humanity. "We ...
Jacobio Receives CDE Approval for Glecirasib's Pancreatic Cancer Pivotal Study in China
BEIJING, SHANGHAI and BOSTON, July 4, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced its novel KRAS G12C inhibitor glecirasib's pivotal study for pancreatic cancer has been approved from CDE (Center for Drug Evalu...
Positive guidance from US FDA on Cu-64 SAR-bisPSMA Phase III trial in prostate cancer
Highlights * FDA agreement for a pivotal Phase III trial for 64Cu SAR-bisPSMA diagnostic in prostate cancer * Phase III trial design based on 64Cu SAR-bisPSMA data package, including positive results from the completed PROPELLER trial * A total of 383 prostate cancer patients to take part ...
China NMPA approves RareStone's pitolisant (Wakix) for the treatment of narcolepsy
-- pitolisant is the first and only treatment drug approved in mainland China for narcolepsy. SHANGHAI, July 4, 2023 /PRNewswire/ -- RareStone Group, a rare disease-focused company aiming to establish the first rare disease ecosystem in China, announced that on June 30, the Chinese National Med...
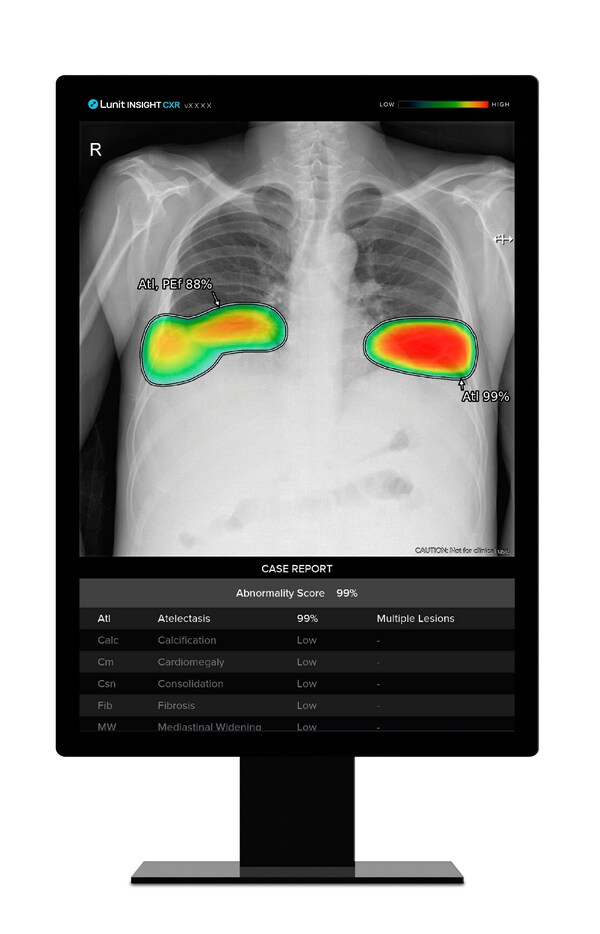
Lunit's AI-Powered Lung Cancer Screening Solution Significantly Affects Radiologists' Diagnostic Determination - Published in Radiology
- Recent study conducted by Seoul National University Hospital provides strong evidence that high-accuracy AI model improves radiologists' chest X-ray analysis performance SEOUL, South Korea, July 3, 2023 /PRNewswire/ -- Findings from a recent study demonstrate that medical AI solutions with onl...
Skyline Therapeutics Receives FDA Clearance of IND for SKG0106, a Novel Intravitreally Delivered AAV Gene Therapy Candidate for Neovascular Age-related Macular Degeneration
SHANGHAI and BOSTON, July 3, 2023 /PRNewswire/ -- Skyline Therapeutics, an innovation-driven gene therapy company committed to developing unique and novel solutions for rare and severe diseases, today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New D...
Henlius Forecasts Profit in 1H 2023
Spurring a Seamless R&D, Manufacturing, and Commercialisation Positive Cycle SHANGHAI, July 3, 2023 /PRNewswire/ -- Henlius (2696.HK) released a positive profit forecast. Based on the preliminary assessment of the unaudited comprehensive management accounts for the six months endedJune 30, 2023, ...
Zymedi announced their collaboration with the National Heart, Lung, and Blood Institute through a CTA CRADA to develop ZMA001 mAb, a potential treatment for Pulmonary Arterial Hypertension (PAH), a rare, female predominant disease
SEOUL, South Korea, July 2, 2023 /PRNewswire/ -- Today Zymedi, a Korean biotech venture-backed company, announced the signing of a clinical Cooperative Research and Development Agreement (CRADA) with the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health ...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00