Health Care/Hospital
Kintor Pharma Announces Publication of Phase Ib Data from Pruxelutamide for AR+ mBC in EJC
SUZHOU, China, Sept. 29, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that the results from a phase Ib study of pruxelutamide (used to be ...
Simcere and Almirall Enter into a Licensing Agreement for IL-2 mu-Fc
* Simcere will receive a $15 million upfront payment, and up to $492 million in development and commercial milestone payments considering successful achievements in several indications, with an important part as sales milestones, as well as up to low double-digit tiered royalties based upon fu...
GC Labs becomes the first Korean laboratory to obtain certification of CDC standardization programs (VDSCP & HoSt)
YONGIN, South Korea and AUSTIN, Texas, Sept. 28, 2022 /PRNewswire/ -- GC Labs, a leadingSouth Korea clinical laboratory, today announced that it has recently obtained certification of standardization programs from the U.S. Centers for Disease Control and Prevention (CDC) for vitamin D and hormone...
Zhongchao Inc. Establishes Chongqing Xinjiang Pharmaceutical Co., Ltd. to Improve Patient Access to Medications
SHANGHAI, Sept. 28, 2022 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), a platform-based internet technology company offering services to patients with oncology and other major diseases, today announced the establishment of Chongqing Xinjiang Pharmaceutical Co., Ltd...
Samsung Biologics Earns Gold Rating from EcoVadis for Its Sustainability Efforts
* Rising from last year's ranking, the company's united effort and dedication to integrating sustainable practices in all areas of its business drew recognition from EcoVadis, which provided high marks in responsible procurement and environmental management. * More than 100,000 companies from...
Portfolio Highlights: Clinical and Financial Updates of ABM, F5, AceLink, HAYA, Regenacy, and Arthrosi
SHANGHAI, Sept. 28, 2022 /PRNewswire/ -- As the investment division of Viva Biotech, Viva BioInnovator is committed to being a collaborative platform for Innovative Biotech companies from around the world. Over the past 2 months, our portfolio companies progressed greatly. ABM Therapeutics Annou...
The 6th China (Shenzhen) Innovation & Entrepreneurship International Competition Eindhoven, Netherlands Division is Open for Registration
DIGITAL ACCESS TO THE WORLD CREATES A BETTER FUTURE EINDHOVEN, Netherlands, Sept. 28, 2022 /PRNewswire/ -- China (Shenzhen)Innovation and Entrepreneurship Competition, known as the "Olympics" of innovation and entrepreneurship circle, is here again! The sixth International competition was offici...
EmeTerm Anti-Nausea Wristband Undergoes Clinical Study for Prevent of Postoperative Nausea and Vomiting
VANCOUVER, BC, Sept. 27, 2022 /PRNewswire/ -- Dr. YongHua Li conducted a clinical trial for EmeTerm, the anti-nausea wristband developed by WAT Medical Enterprise, Ltd.,Vancouver, BC, Canada. The study spanned across three hospitals and was aimed to investigate EmeTerm's effectiveness in its tra...
Harbour BioMed Announces First Subject Dosed in Phase I Study of Next-Gen Anti-TSLP Fully Human Monoclonal Antibody
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Sept. 27, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) today announces that it has completed the first subject dosing in a phase I study of HBM9378 (or SKB378 as referred to by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd...
Neurophth announces first patient dosed in Phase III clinical trial for the gene therapy treatment of LHON
WUHAN, China and SAN DIEGO, Sept. 27, 2022 /PRNewswire/ -- Neurophth Therapeutics, Inc. ("Neurophth") announced today thatthe first patient has been dosed in Phase III clinical trial for the treatment of Leber hereditary optic neuropathy (LHON). Neurophth is conducting a Phase I/II/III, multi-...
Angel Pharmaceuticals Announces Approval of IND Application for Phase 1/1b Clinical Trial of Mupadolimab (Anti-CD73) in China
JIAXING,China and BURLINGAME,Calif., Sept. 26, 2022 /PRNewswire/ -- Angel Pharmaceuticals Ltd. ("Angel Pharma"), a clinical-stage biopharmaceutical company,today announced that the IND application for mupadolimab (formerly CPI-006) has been approved by the Center for Drug Evaluation (CDE) to init...
Fosun Pharma and MAIA Pharmaceuticals Jointly Announce the Successful Commercialization of Only FDA Approved 20ml Sodium Phenylacetate and Sodium Benzoate (SPSB) Liquid Product
PRINCETON, N.J., Sept. 26, 2022 /PRNewswire/ -- Shanghai Fosun Pharma (Group) Co., Ltd. ("Fosun Pharma", stock code: 600196.SH; 02196.HK) today announced that the 20 ml, Sodium Phenylacetate and Sodium Benzoate (SPSB) liquid product, which is under an exclusive partnership betweenFosun Pharma USA...
Bioheng Biotech Announces Publication of Impressive Results with CD7-targeted allogeneic CAR-T cell therapy for Relapsed or Refractory T Cell Malignancies
NANJING and HANGZHOU, China, Sept. 26, 2022 /PRNewswire/ -- Nanjing Bioheng Biotech Co., Ltd, a clinical-stage biotechnology company focused on developing novel cellular immunotherapy, today announced that a phase I clinical study results of RD13-01, an anti-CD7 universal CAR-T therapy product, h...
PharmAbcine to Participate in BIO-Europe 2022
DAEJEON, South Korea, Sept. 26, 2022 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next generation antibody therapeutics, announced today that the Company will participate in the upcoming BIO-Europe 2022 which will take place ...
WuXi STA Opens First High Potency Oral Drug Product Manufacturing Facility
New facility expands integrated drug product R&D and manufacturing services to global customers SHANGHAI, Sept. 26, 2022 /PRNewswire/ -- WuXi STA, a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), today announced the opening of its first high potency (HP) o...
Alterity Therapeutics Announces Presentation of Wearable Sensor Data from the bioMUSE Natural History Study at the International Congress of Parkinson's Disease and Movement Disorders
- Wearable sensors strongly correlated with clinical scales on motor impairment - - bioMUSE Study Validates Use of Wearable Sensors for Alterity's Ongoing Phase 2 Clinical Trial - MELBOURNE, Australia and SAN FRANCISCO, Sept. 26, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATH...
CellOrigin Biotech Announces Global Strategic Collaboration with Qilu Pharma to Develop "Off-the-Shelf" CAR-iMAC Cell Therapy
HANGZHOU, China, Sept. 26, 2022 /PRNewswire/ -- CellOrigin Biotech (Hangzhou) Co., Ltd. announced it has made a global strategic collaboration agreement with Qilu Pharma to develop, manufacture and commercialize proprietary "off-the-shelf" induced pluripotent stem cell- (iPSC) derived Chimeric An...
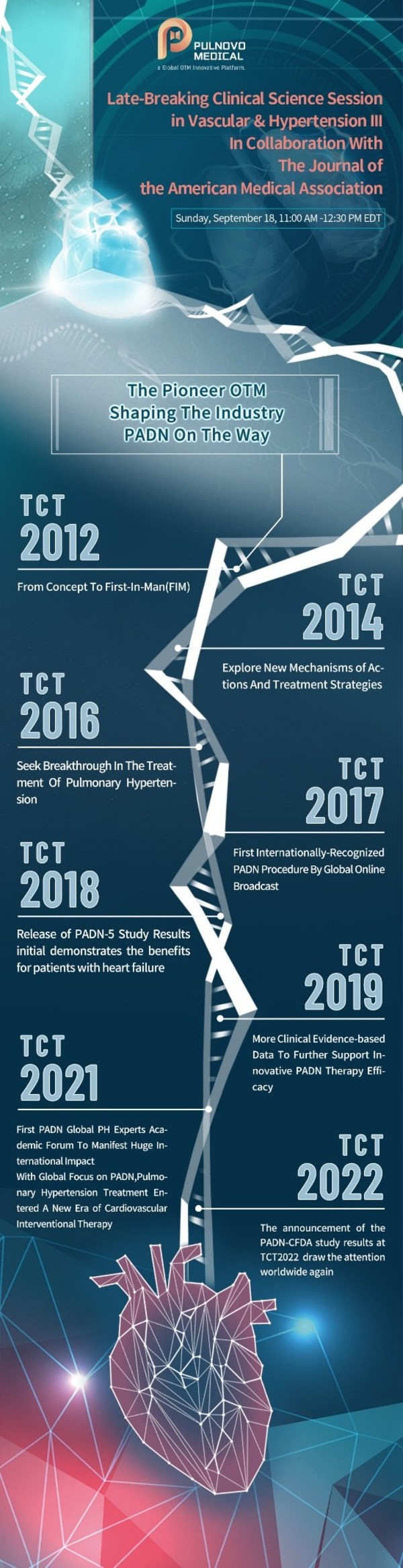
TCT2022| Pulnovo Medical Announced That The Latest Results Of PADN-CFDA Pivotal Trial Indicate Effectiveness And Safety of PADN For The Treatment Of Pulmonary Arterial Hypertension (PAH)
SHANGHAI, Sept. 23, 2022 /PRNewswire/ -- Pulnovo Medical Limited, a globally recognized OTM platform, today announced the positive results from the Pulmonary Artery Denervation (PADN)-CFDA pivotal study on the world's largest and most influential American transcatheter cardiovascular therapy conf...
'iSyncWave' EEG Scanner receives FDA 510k clearance
SEOUL, South Korea, Sept. 23, 2022 /PRNewswire/ -- iMediSync announced that
iSyncWave, the company's medical device EEG scanner, has been granted510k
clearance from the US Food and Drug Administration (FDA) onAugust 10th.
111, Inc. Announces Appointment of Independent Financial Advisor and Legal Counsel to the Special Committee
SHANGHAI, Sept. 23, 2022 /PRNewswire/ -- 111, Inc. ( "111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company in China, announced today that the special committee (the "Special Committee"), consisting of three independent directors, Mr.Jian Sun, who is the chairman...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 295 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 293 media titles]
2024-11-26 13:00