Health Care/Hospital
Happiness Development Exported $1.2 million Lucidum Products
NANPING, China, Dec. 6, 2021 /PRNewswire/ -- Happiness Development Group Limited ("HAPP" or the "Company"),(NASDAQ: HAPP) a China-based company engaging in the business of production of nutraceutical and dietary supplements, providing e-commerce solutions, and the sales of automobile, announced t...
IMPACT 2021 R&D Day: Synthetic Lethality Provides a New Platform for Targeted Cancer Therapy
SHANGHAI, Dec. 6, 2021 /PRNewswire/ -- The 2nd R&D Day event of IMPACT Therapeutics (IMPACT) successfully took place inShanghai on December 3rd (GMT+8). With the theme of "Synthetic Lethality: A New Platform for Targeted Therapy", the event attracted nearly 300 guests offline and online. The gues...
Odyssey Announces Agreement to Combine with BenevolentAI
* BenevolentAI is a leading, clinical-stage AI drug discovery company that combines advanced AI and machine learning with cutting edge science to discover and develop novel and more effective medicines * Combination of BenevolentAI with Odyssey, a €300m Euronext Amsterdam-listed special purpo...
Jacobio Receives IND Approval for Combination Therapy of KRAS G12C and Cetuximab Injection in China
BEIJING and SHANGHAI and BOSTON, Dec. 5, 2021 /PRNewswire/ -- Jacobio Pharma ( 1167.HK) has received the Investigational New Drug (the "IND") approval of the combination therapy of KRAS G12C inhibitor JAB-21822 and Cetuximab injection from the Center for Drug Evaluation ofChina (the "CDE") on Dec...
Kazia Announces Positive Final Data From Phase II Clinical Study Of Paxalisib In Newly Diagnosed Glioblastoma
SYDNEY, Dec. 4, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce positive final data from a phase II clinical study of paxalisib as first line therapy in patients with glioblastoma (NCT03522298). The res...
I-Mab Announces First Two Patients Dosed in U.S. Phase 2 Combination Trial of Uliledlimab with Atezolizumab in Patients with Selected Advanced Solid Tumors
SHANGHAI and GAITHERSBURG, Md., Dec. 3, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the first two patients have been dosed in the U.S. p...
OrigiMed announced its strategic partnership with Janssen to develop clinical innovative solutions driven by data science
SHANGHAI, Dec. 3, 2021 /PRNewswire/ -- OrigiMed Co., Ltd. ("OrigiMed") announced that the company has signed a memorandum of understanding (MoU) on strategic collaboration with Janssen (China) Research and Development Center of Johnson & Johnson (China) Investment Co., Ltd. ("Janssen").Zili Li, M...
Biotheus' Bifunctional Therapeutic PM8001 has been Approved by the USFDA to Enter Phase II Studies
ZHUHAI, China, Dec. 3, 2021 /PRNewswire/ -- Biotheus Inc., has announced that its proprietary anti-PD-L1/TGF-β bifunctional therapeutic, named PM8001, has been approved by the USFDA (United States Food and Drug Administration) for conducting clinical phase II studies as part of an international m...
InnoCare Announces Inclusion of Orelabrutinib in China National Reimbursement Drug List
BEIJING, Dec. 2, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a commercial-stage biotech company, announced today that its BTK inhibitor orelabrutinib has been included in the updated National Reimbursement Drug List (NRDL) by the China National Healthcare Security Administration (NHSA). ...
Hepagene Therapeutics Initiates the RISE Study, a Phase IIa Clinical Trial of HPG1860 in Patients with NASH
SHANGHAI, Dec. 2, 2021 /PRNewswire/ -- Hepagene Therapeutics, Inc, a clinical stage biopharmaceutical company focusing on innovative therapies for patients with liver diseases, today announced that it has screened the first patient in theUSA for the RISE study, a Phase IIa clinical trial of HPG18...
MingMed Biotechnology Announces U.S. FDA Approval for IND Application of HPK1 Small Molecule Inhibitor PRJ1-3024, and Completion of Phase I Clinical Trials for Dry AMD Treatment Drug QA102
GUANGZHOU, China, Dec. 2, 2021 /PRNewswire/ -- MingMed Biotechnology, a clinical stage company dedicated to developing first-in-class pharmaceutical products, announced recently the US Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for PRJ1-3024, a ...
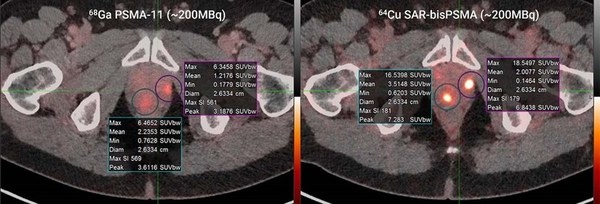
Clarity and Cardinal Health enter into Agreement for Targeted Copper Theranostics
SYDNEY, Dec. 2, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, and Cardinal Health (NYSE: CAH), are pleased to announce that the companies have entered ...
Rayence's Low-Dose Detector "GreenON" Receives Critical Acclaim at RSNA 2021
SEOUL, South Korea, Dec. 2, 2021 /PRNewswire/ -- Rayence(www.rayence.com
DiNovA Medtech Announces Mr. Yaniv Kirma to Join as DiNovA Israel Incubator's Chief Operating Officer
CAESAREA, Israel, Dec. 2, 2021 /PRNewswire/ -- DiNovA Medtech, a leading medical devices incubator inChina ("DiNovA"), is pleased to announce Mr. Yaniv Kirma, to come on board as Chief Operating Officer of DiNovA Israel Incubator ("DiNovA Israel"). Mr. Kirma will join force with Chief Medical Off...
Fifty percent recruitment milestone for PROPELLER prostate cancer trial
SYDNEY, Dec. 1, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that 15 of 30 participants have been recruited in the diagnostic64...
Licensing Partner of Shenzhen Chipscreen Biosciences - HUYABIO International, Receives Regulatory Approval for Chidamide Monotherapy of Peripheral T-Cell Lymphoma(PTCL) in Japan
SHENZHEN, China, Dec. 1, 2021 /PRNewswire/ -- Shenzhen Chipscreen Biosciences' licensing partner, HUYABIO International (HUYABIO™), today announced the regulatory approval for Chidamide (Tucidinostat, also known as Epidaza ®, Hiyasta®, HBI-8000) monotherapy for the treatment of relapsed or refrac...
DEVITA Global joins hands with Bodi Insurance Group, Mongolia's premier insurance provider, to advance a decentralized health data sharing economy.
SEOUL, South Korea, Dec. 1, 2021 /PRNewswire/ -- DEVITA Global ("DEVITA") together with the Bodi Insurance Group ("Bodi Insurance") have agreed on a cooperation partnership for the joint development of a decentralized health data sharing economy by employing blockchain technology to create a netw...
U.S. FDA Grants Bionomics Fast Track Designation to BNC210 for the Acute Treatment of Social Anxiety Disorder and Other Anxiety Related Disorders
ADELAIDE, Australia, Dec. 1, 2021 /PRNewswire/ -- Bionomics Limited (ASX:BNO, OTCQB:BNOEF) (Bionomics or Company), a clinical-stage biopharmaceutical company, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to the BNC210 development progr...
Great Bay Bio Completes a Near USD 10M Series A Fundraising
HONG KONG, Dec. 1, 2021 /PRNewswire/ -- Great Bay Bio (hereinafter referred to as "GBB") is pleased to announce today the completion of its nearUSD 10M series A fundraising led by Proxima Ventures. The round was oversubscribed and upsized with participation by many new and existing institutional ...
Binhai New Area to spearhead China's medical industry development
TIANJIN, China, Nov. 30, 2021 /PRNewswire/ -- Here's a news report from chinadaily.com.cn: By 2025, the Binhai New Area in North China's Tianjin municipality will cultivate industry clusters worth tens of billions of yuan that are dedicated to pharmaceuticals, biotech, medical devices, diagnosti...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 298 media titles]
2024-11-28 17:24Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00Mentech at COP29: Showing the Eco-friendly Lifestyle with Technological Innovation
[Picked up by 281 media titles]
2024-11-29 20:33MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00