Medical/Pharmaceuticals
Minghui Pharmaceutical Inc. Announces Positive Topline Results from Phase Ib/II Clinical Trial of MHB018A, a Subcutaneous Single-Domain IGF-1R Antibody, in Patients with Active, Moderate-to-Severe Thyroid Eye Disease (TED)
SHANGHAI, Oct. 22, 2024 /PRNewswire/ -- Minghui Pharmaceutical, Inc., a late-stage biopharmaceutical company dedicated to developing transformative medicines in immunology and oncology, today announced positive topline results from its Phase Ib/II clinical trial of MHB018A in patients with active...
HELP Therapeutics announces FDA clearance of IND application for universal iPSC-derived HiCM-188 cell therapy for the treatment of end-stage heart failure
NANJING, China, Oct. 22, 2024 /PRNewswire/ -- HELP Therapeutics Co. Ltd, a clinical-stage cell therapy company,today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for "Allogeneic Human iPSC-derived cardiomyocytes (HiCM-188) a...
South Korean Mental Tech Startup DoctorPresso Publishes Study to Detect Depression from User-Generated Diary test data in International Journal
SEOUL, South Korea, Oct. 21, 2024 /PRNewswire/ -- Doctorpresso's study to detect depression from user-generated diary text data was published in the September 2024 issue of the Journal of Medical Internet Research (JMIR) in recognition of its excellence. JMIR noted the "new approach of Doctorpress...
A Comprehensive Introduction of the oneClick+ antigen platform
SHANGHAI, Oct. 21, 2024 /PRNewswire/ -- Sanyou Bio provides oneClick+ antigen one-stop solution, from antigen analysis to personalized preparation, to help tumor immunotherapy research and development. oneClick+ antigen platform is designed to provide high-quality annotation-related protein seque...
Datasea Announces the Closing of a $4.0 Million Private Offering, Primarily Funded by the Company's CEO and Director
BEIJING, Oct. 21, 2024 /PRNewswire/ -- Datasea Inc. (NASDAQ: DTSS) ("Datasea" or the "Company"), aNevada-based digital technology company focused on innovative high-tech acoustics and 5G AI multimodal digital technology, today announced that, as ofOctober 15, 2024, the Company closed its Regulati...
Mediso introduces true theranostic TheraMAX SPECT/CT
HAMBURG, Germany, Oct. 21, 2024 /PRNewswire/ -- Mediso is unveiling its new
AnyScan® TRIO SPECT/CT, TheraMAX* scanner at the annual meeting of European
Association of Nuclear Medicine (EANM).
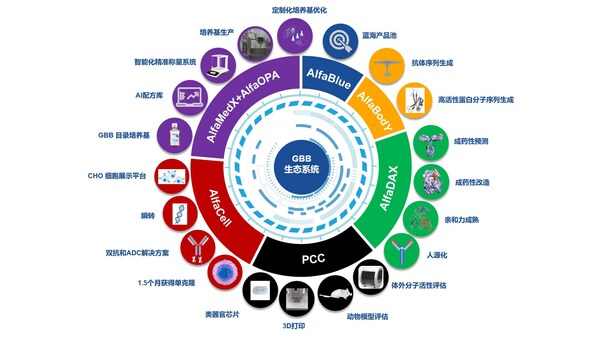
Great Bay Bio Intelligent Drug Development Ecosystem Officially Launched
HONG KONG, Oct. 20, 2024 /PRNewswire/ -- On October 18th, the new product launch event hosted by Great Bay Bio (hereinafter referred to as "GBB") was successfully held in Suzhou, where industry players gathered to celebrate the feast of technological innovation. At the event, GBB announced the of...
MicuRx Pharmaceuticals Showcases the Pipeline Status of MRX-5 and MRX-8 at BIO Investor Forum
SAN FRANCISCO, Oct. 18, 2024 /PRNewswire/ -- Dr. Regis Vilchez, Chief Medical Officer of MicuRx Pharmaceuticals, presented the latest advancements of company's development program, MRX-5 and MRX-8, at the BIO Investor Forum (BIF). These development programs offer new potentials for the future tre...
Neurophet to host Symposium featuring renowned expert in Alzheimer's disease treatment from the U.S.
- Invited Neurophet's scientific advisor Stephen Salloway, professor of Neurology at Brown Medical School as guest speaker - Shared latest insights on brain image analysis used for prescription of Alzheimer's disease treatments such as 'Leqembi' and 'Kisunla' SEOUL, South Korea, Oct. 18, 2024 /P...
LaNova Medicines Announces Initiation of Phase 1 Clinical Trial of Anti-PD-1/VEGF Bispecific Antibody LM-299 and Completion of $42 Million Series C1 Financing
* Phase 1 trial of LM-299, an anti-PD-1/VEGF BsAb, initiated in China for advanced solid tumors following promising preclinical results demonstrating strong inhibition of tumor growth and well-tolerated safety profile * IND for LM-299 in the US expected to be submitted in the second half of 20...
111 to Announce Third Quarter 2024 Unaudited Financial Results on November 28, 2024 - Conference Call to Follow
SHANGHAI, Oct. 18, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to reshaping the value chain of healthcare industry by digitally empowering the upstream and downstream inChina, today announced that it will repor...
Ping An Health Upgrades Chronic Disease Management Services with Significant Results in Digital Weight Management Program
HONG KONG and SHANGHAI, Oct. 18, 2024 /PRNewswire/ -- The Weight Management and Public Health Action Conference, hosted by the National Center for Chronic and Noncommunicable Disease Control and Prevention ("NCNCD") and its affiliated institution, the Binhai Institute for Chronic Disease Control ...
Exclusive Interview with Lang Guojun, founder of Sanyou Bio: The Key to Breaking the Bottleneck of New Drug Innovation--The Past and Future of the Super-Trillion Molecule Library
SHANGHAI, Oct. 17, 2024 /PRNewswire/ -- In the ninth year since the establishment of Sanyou Bio, Lang Guojun believes that the time is perfect to unveil the world's largest super-trillion molecule library. During the interview with Tongxieyi, he frankly and confidently expressed, "Nine years of ...
Chipscreen Biosciences' Innovative Anti-Tumor Drug CS231295 Tablets: Investigational New Drug (IND) Application Accepted
SHENZHEN, China, Oct. 17, 2024 /PRNewswire/ -- On October 17, 2024, Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) submitted the company's Investigational New Drug (IND) application for CS231295 tablets, a Class 1 innovative drug for the treatment of t...
Amplo Biotechnology Announces Three Presentations at the European Society of Gene and Cell Therapy 31st Annual Congress
SAN DIEGO, Oct. 17, 2024 /PRNewswire/ -- Amplo Biotechnology, a biotechnology company developing genetic medicines for the treatment of neuromuscular diseases, announced the presentation of three posters at the European Society of Gene and Cell Therapy (ESGCT) 31st Annual Congress, taking place f...
KIND Announces Late-Breaking Abstract Accepted for Presentation on AND017 Clinical Trials to Treat Anemia in Chronic Kidney Disease (CKD) at ASN - Kidney Week 2024
SAN FRANCISCO, Oct. 17, 2024 /PRNewswire/ -- Kind Pharmaceutical ("Hangzhou Andao Pharmaceutical Ltd. and Kind Pharmaceuticals LLC"), a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, today announced that the late-br...
EmeTerm Smart and HeadaTerm 2 Achieve Health Canada MDL Certification
VANCOUVER, BC, Oct. 16, 2024 /PRNewswire/ -- WAT Medical Enterprise proudly announces a significant achievement: both EmeTerm Smart and HeadaTerm 2 have received Medical Device Licences (MDL) from Health Canada. EmeTerm Smart was certified onAugust 12, 2024, followed by HeadaTerm 2 on September 2...
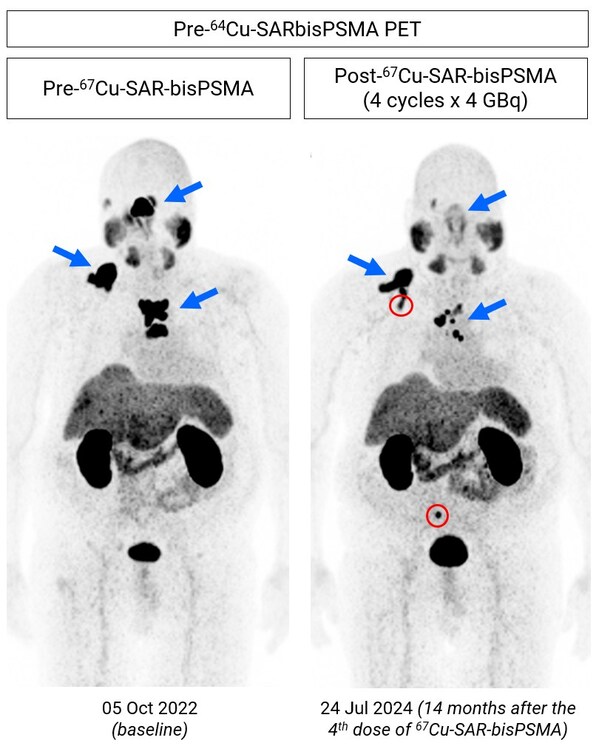
Copper-67 SAR-bisPSMA updates
SYDNEY, Oct. 16, 2024 /PRNewswire/ -- HIGHLIGHTS Cohort 4 - SECuRE Trial * The third participant of cohort 4 (multi-dose) of the SECuRE trial1 has now completed the Dose Limiting Toxicity (DLT) period after a second dose of 12GBq of67Cu-SAR-bisPSMA, following on from the announcement dated 1...
IGCS Late Breaking Abstract and The Lancet: Akeso Published Positive PFS and OS Results from Phase 3 First-Line Study of Cadonilimab in Cervical Cancer
HONG KONG, Oct. 16, 2024 /PRNewswire/ -- Akeso Biopharma (9926.HK) (" Akeso", the "Company" ) announced positive results in progression-free survival (PFS) and overall survival (OS) from its Phase 3 clinical study (COMPASSION-16/AK104-303). This study evaluated the efficacy of its independently...
TenNor Announces More than 300 Million RMB Financing to Support Development and Commercialization of Late-Stage Assets Including Rifasutenizol for Heliobacter pylori Infections
SUZHOU, China, Oct. 16, 2024 /PRNewswire/ -- TenNor Therapeutics, a clinical stage company dedicated to developing new therapies to address unmet needs in infectious diseases, announced today the initial closing of a Series E financing round for more than300 million RMB. New investor AMR Action F...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 317 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00