Medical/Pharmaceuticals
Planet Innovation acquires BIT Group's North American operations, doubling its medtech manufacturing capacity
MELBOURNE, Australia, Nov. 11, 2021 /PRNewswire/ -- Planet Innovation (PI) has acquired the North American operations of global regulated medical device manufacturer, BIT Analytical Instruments GmbH. The acquisition solidifies PI's commitment to grow its US manufacturing capabilities to suppor...
Tabernacle Health Group Launches Revolutionary Immunity Booster. Natural Health Supplement Avrocil (TM) Treats Cold and Flu Symptoms caused by Upper Respiratory Tract Infections
SINGAPORE, Nov. 11, 2021 /PRNewswire/ -- The pandemic sped up participation in virtual exercise classes acrossthe United States, as countless fitness enthusiasts switched to indoor workouts to avoid viruses, such as those that lead to accelerated upper respiratory tract infections, whose sympto...
Patients benefited from telemedicine during COVID-19, says new white paper from Healint
* Longitudinal surveys conducted via HeRO™, Healint's proprietary real-world patient insights platform, reveal that 84% would choose telemedicine if it was available, with a preference for follow-up consultations * A 9-month follow-up questionnaire was sent to evaluate changes in patients' ex...
Lunit Presents Studies at SITC 2021, Highlighting the Effectiveness of AI in Predicting Response to Immunotherapy in a Clinical Trial Setting
- Lunit to present three abstracts about its AI biomarker platform Lunit SCOPE IO, also to be demonstrated during the event in booth #423 - One immunotherapy combination study adds significant evidence for the potential value of using Lunit SCOPE IO in clinical practice to predict patient res...
PNOC Study in Childhood Brain Cancer Enrols First Patient
SYDNEY, Nov. 11, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that PNOC022 (NCT05009992), a multi-drug phase II study in DIPG and diffuse midline gliomas, has been initiated at theUniversity of Calif...
Gmax's GMA106, second generation obesity/T2DM/NASH mAb gives first in human dose
HANGZHOU, China, Nov. 11, 2021 /PRNewswire/ -- Gmax Biopharm today announces that the first dose of GMA106 was given to human subjects in a phase 1 study to investigate the safety, pharmacokinetics, and pharmacodynamics of this drug in the treatment of obesity. The study is a single dose, placeab...
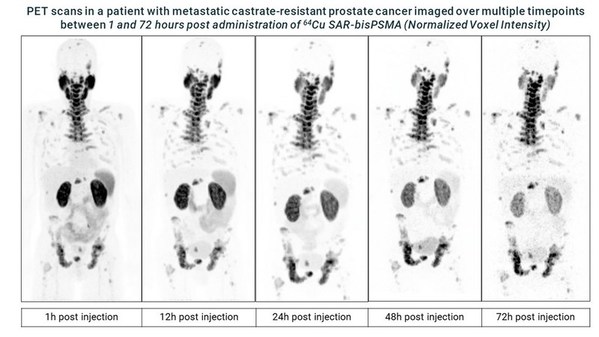
Recruitment for the dosimetry phase of Clarity's Cu-64/Cu-67 SAR-bisPSMA theranostic prostate cancer trial completed
* Clarity Pharmaceuticals completes recruitment for the initial dosimetry phase of its SAR-bisPSMA theranostic clinical trial SECuRE (NCT04868604)[1] investigating Targeted Copper Theranostics (TCT) in patients with metastatic castrate-resistant prostate cancer (mCRPC). * Dosimetry data is be...
IASO Biotherapeutics and Innovent Biologics to Present New BCMA CAR-T Cell Therapy Data in Oral Presentation at ASH 2021
SAN JOSE, Calif., NANJING, China and SHANGHAI, Nov. 10, 2021 /PRNewswire/ --
IASO Biotherapeutics("IASO Bio")
I-Mab and Jumpcan Announce Strategic Commercial Partnership on Eftansomatropin Alfa
* The partnership brings together the strengths of an innovative global biotech and a domestic leading pharmaceutical company specialized in and committed to pediatric medicines to accelerate the commercialization of eftansomatropin alfa * Jumpcan will pay I-Mab for a total of up to RMB 2.016...
Hummingbird Bioscience Announces Trials in Progress Poster Presentation at the Society for Immunotherapy of Cancer 2021 36th Annual Meeting
HOUSTON, Nov. 10, 2021 /PRNewswire/ -- Hummingbird Bioscience, an innovative clinical-stage biotech company focused on developing precision therapies against hard-to-drug targets, today announced a Trials in Progress poster presentation outlining the Phase 1 clinical trial design for HMBD-002, a ...
AffaMed Therapeutics Announces the Establishment of AffaMed Digital to Advance Digital Medicines
SHANGHAI, Nov. 10, 2021 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical-stage therapeutic company dedicated to developing and commercializing transformative pharmaceutical, digital and surgical products that address critical unmet medical needs in ophthalmic, neurological and ...
OMRON Launches World's Fastest CT X-ray Inspection with New VT-X750-V3 AXI System
KYOTO, Japan, Nov. 10, 2021 /PRNewswire/ -- OMRON Corporation based in Kyoto, Japan, has announced the development of a new VT-X750-V3 system, the world's fastest (*1) CT-type X-ray inspection device (*2) to date, and said it will be released globally onNovember 20. The VT-X750-V3 delivers advanc...
I-Mab and ABL Bio Report Preclinical Data of 4-1BB-targeting Bispecific Antibodies at 2021 SITC
* Preclinical data of TJ-CD4B/ABL111 and TJ-L14B/ABL503 demonstrate targeted safety profile and enhanced anti-tumor activity * Both studies are undergoing phase 1 clinical trials in the United States SHANGHAI and GAITHERSBURG, Md. and SEONGNAM, South Korea, Nov. 9, 2021 /PRNewswire/ -- I-Mab ...
Alterity Therapeutics Announces Presentation of ATH434 at the American Autonomic Society Virtual Meeting 2021
MELBOURNE, Australia, Nov. 9, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative conditions, today announced a poster presentation and accompanying vi...
FDA Clears Pharmaxis Cancer Drug to Progress to Phase 2 Study in Liver Cancer
SYDNEY, Nov. 9, 2021 /PRNewswire/ -- Clinical stage drug development company Pharmaxis Ltd (ASX: PXS) today announced that an Investigational New Drug application (IND) for a trial of PXS-5505 in hepatocellular carcinoma (HCC) patients has been cleared by the United States Food and Drug Administr...
Standigm Established a Synthetic Research Center to Improve Efficiency of AI Drug Discovery
SEOUL, South Korea, Nov. 8, 2021 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, announced today that the company had established a Synthetic Research Center in the headquarters of SK Chemicals Co., Ltd ("SK Chemicals", KRX 285...
BIORCHESTRA will present data on the therapeutic efficacy of their proprietary ASO against pathological miRNA, a key modulator of neuroinflammation and neurodegeneration, at the CTAD Meeting in Boston
BOSTON, Nov. 5, 2021 /PRNewswire/ -- South Korean bio-venture BIORCHESTRA Co. Ltd., is an RNA therapeutics firm focused on neurodegeneration. BIORCHESTRA discovered a disease-associated microRNA that was found to be significantly up-regulated in samples from Alzheimer's disease patients. It then...
Ascentage Pharma Announces Clinical Trial Agreement to Evaluate the Combination of Lisaftoclax (APG-2575) and the CDK4/6 Inhibitor IBRANCE® (Palbociclib) in Metastatic ER+/HER2- Breast Cancer
SUZHOU, China, and ROCKVILLE, Md., Nov. 8, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced a clinical trial collaborati...
I-Mab and Roche Diagnostics Announce Strategic Collaboration to Co-Develop Companion Diagnostics Solutions for I-Mab's Innovative Pipeline at the 4th CIIE
SHANGHAI and GAITHERSBURG, Md., Nov. 8, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the Company has entered into a strategic collaborati...
Everest Medicines Announces Up to HK$100 million Additional Share Repurchase Program
SHANGHAI, Nov. 7, 2021 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest" or the "Company"), a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products that address critical unmet medical needs for patients inAsia, today announced that i...
Week's Top Stories
Most Reposted
Agoda releases generative AI film to capture the joy of travel
[Picked up by 348 media titles]
2024-12-10 10:00Singapore's IMAGINE AI: Largest Global Gathering to Shape the Future of Healthcare with AI Innovations
[Picked up by 316 media titles]
2024-12-06 10:41Mastercard Economics Institute: APAC growth expected to hold steady in 2025; global policy resets may see shift in gears
[Picked up by 313 media titles]
2024-12-10 09:00Building Asia's Future: Omni HR Secures $7.4mn for HR Tech Expansion
[Picked up by 306 media titles]
2024-12-09 10:00CCTV+: Yuhang Journey ǀ Follow Olivier to Explore Ancient Liangzhu and Savor Jingshan Tea
[Picked up by 283 media titles]
2024-12-08 20:07