Medical/Pharmaceuticals
FDA Clears Pharmaxis Cancer Drug to Progress to Phase 2 Study in Liver Cancer
SYDNEY, Nov. 9, 2021 /PRNewswire/ -- Clinical stage drug development company Pharmaxis Ltd (ASX: PXS) today announced that an Investigational New Drug application (IND) for a trial of PXS-5505 in hepatocellular carcinoma (HCC) patients has been cleared by the United States Food and Drug Administr...
Standigm Established a Synthetic Research Center to Improve Efficiency of AI Drug Discovery
SEOUL, South Korea, Nov. 8, 2021 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, announced today that the company had established a Synthetic Research Center in the headquarters of SK Chemicals Co., Ltd ("SK Chemicals", KRX 285...
BIORCHESTRA will present data on the therapeutic efficacy of their proprietary ASO against pathological miRNA, a key modulator of neuroinflammation and neurodegeneration, at the CTAD Meeting in Boston
BOSTON, Nov. 5, 2021 /PRNewswire/ -- South Korean bio-venture BIORCHESTRA Co. Ltd., is an RNA therapeutics firm focused on neurodegeneration. BIORCHESTRA discovered a disease-associated microRNA that was found to be significantly up-regulated in samples from Alzheimer's disease patients. It then...
Ascentage Pharma Announces Clinical Trial Agreement to Evaluate the Combination of Lisaftoclax (APG-2575) and the CDK4/6 Inhibitor IBRANCE® (Palbociclib) in Metastatic ER+/HER2- Breast Cancer
SUZHOU, China, and ROCKVILLE, Md., Nov. 8, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced a clinical trial collaborati...
I-Mab and Roche Diagnostics Announce Strategic Collaboration to Co-Develop Companion Diagnostics Solutions for I-Mab's Innovative Pipeline at the 4th CIIE
SHANGHAI and GAITHERSBURG, Md., Nov. 8, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the Company has entered into a strategic collaborati...
Everest Medicines Announces Up to HK$100 million Additional Share Repurchase Program
SHANGHAI, Nov. 7, 2021 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest" or the "Company"), a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products that address critical unmet medical needs for patients inAsia, today announced that i...
PromarkerD significantly outperforms current standard of care tests in predicting future kidney function decline
* Study shows PromarkerD is significantly better than current standard of care tests eGFR and ACR for predicting future decline in kidney function in patients with type 2 diabetes * PromarkerD correctly identified 84% of patients with normal kidney function that went on to experience kidney f...
Alterity Therapeutics Announces New Publications Providing Further Evidence of the Potential of ATH434 to Treat Neurodegenerative Diseases
MELBOURNE, Australia, Nov. 5, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative conditions, today announced the publication of two preclinical studie...
ASH 2021 | Ascentage Pharma to Release Latest Data from Two Studies of Its Bcl-2-Selective Inhibitor Lisaftoclax (APG-2575), Including a Chinese Study Demonstrating Complete Response
SUZHOU, China, and ROCKVILLE, MD., Nov. 4, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that abstracts on six studies of the company's thr...
ASH 2021 | Ascentage Pharma to Present Three Studies of Olverembatinib (HQP1351), a Novel Drug Candidate for the Treatment of Drug-Resistant Leukemia, in Abstracts Including One Oral Report at ASH Annual Meeting
SUZHOU, China and ROCKVILLE, MD., Nov. 4, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that abstracts on three clinical trials of the c...
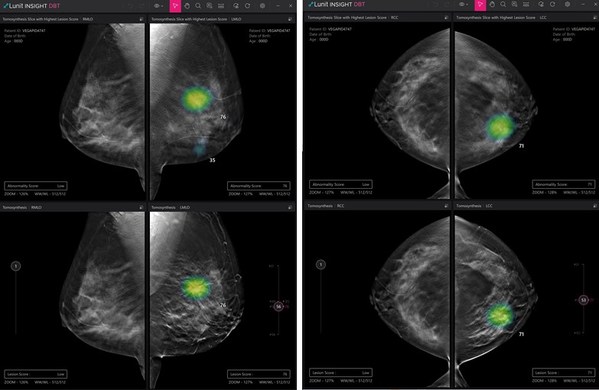
Lunit to Participate in RSNA 2021, Presenting its New AI Solutions for Digital Breast Tomosynthesis and Chest CT
SEOUL, South Korea, Nov. 4, 2021 /PRNewswire/ -- Lunit, a leading medical AI provider, will be returning to the 107th Radiological Society ofNorth America (RSNA) this year with its up-to-date, comprehensive AI solutions for chest and breast radiology. Along with its most mature products, the comp...
Gracell Biotechnologies to Present Preclinical Results of TruUCAR-enabled CD19/CD7 Dual-Directed Allogeneic CAR-T Cell Therapy (GC502) for B-Cell Malignancies at the 63rd ASH Annual Meeting
SUZHOU and SHANGHAI, China, and PALO ALTO, California, Nov. 4, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, toda...
I-Mab to Present Clinical Data of Lemzoparlimab in Combination with Rituximab in Non-Hodgkins's Lymphoma at ASH 2021
* Lemzoparlimab is a differentiated CD47 monoclonal antibody discovered by I-Mab and being developed in collaboration with AbbVie * Initial clinical results of lemzoparlimab in combination with rituximab in NHL will be presented at the ASH 2021 Annual Meeting * I-Mab to host a call for inves...
Taizhou, China: Building a Famous Chinese Medical City with Efforts
TAIZHOU, China, Nov. 4, 2021 /PRNewswire/ -- Recently, the 12th China (Taizhou) International Medical Expo was held inChina Medical City in Taizhou, China. Starting from the needs of normalized prevention and control of the epidemic, this year's expo made an "online+on-site" integrated exhibition...
Medigate Accelerates Expansion to Meet Growing Demand in Asia-Pacific Region
Healthcare's IoT cybersecurity and clinical asset management leader names APAC Regional Director. BROOKLYN, N.Y., Nov. 4, 2021 /PRNewswire/ -- Medigate, Healthcare's Security and Clinical Asset Management leader, today announced its expansion into the Asia-Pacific (APAC) region. Capitalizing on ...
Alterity Therapeutics Announces New Publications Providing Further Evidence of the Potential of ATH434 to Treat Neurodegenerative Diseases
MELBOURNE, Australia, Nov. 4, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative conditions, today announced the publication of two preclinical studie...
Medtecs to showcase PPE and technologies from Taiwan at MEDICA 2021
TAIPEI, Nov. 3, 2021 /PRNewswire/ -- It is now half-way through autumn and the COVID-19 pandemic still persists. The global death toll has reached over 5 million yet only 45.4% of the global population has received at least one dose of vaccine. To make matters worse, a new subvariant of the virus...
Gold Technology for Immune Response Cancer Therapy Licensed to OncoTEX
AUSTIN, Texas, Nov. 3, 2021 /PRNewswire/ -- OncoTEX Inc. (OncoTEX), a US oncology company and member of The iQ Group Global portfolio, is pleased to announce it has acquired a gold compound platform technology that induces the body's immune system to destroy cancer cells. The technology has been...
TransThera Receives Fast Track Designation from FDA for its Core Product TT-00420 to Treat Cholangiocarcinoma
NANJING, China, Nov. 3, 2021 /PRNewswire/ -- TransThera Sciences (Nanjing) Inc., a clinical-stage biopharmaceutical company focusing on discovering and developing innovative small molecule drug therapies for oncology, inflammatory and cardiovascular diseases, announced that the U.S. Food and Drug...
Standigm Files PCT Patent Application of AI-driven Repurposed Drugs for Primary Mitochondrial Disease
SEOUL, South Korea, Nov. 3, 2021 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, announced today that the company had filed PCT ("Patent Cooperation Treaty") patent application covering AI-driven repurposed drugs for primary m...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 299 media titles]
2026-02-09 10:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 299 media titles]
2026-02-10 13:31Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 296 media titles]
2026-02-11 11:00