Medical/Pharmaceuticals
Kintor Pharmaceuticals Announced Successful Dosing of the First Batch of Patients for Acne Vulgaris Phase I/II Clinical Trial of Pyrilutamide
SUZHOU, China, April 16, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939), a clinical-stage biotechnology company developing small molecule and biological therapeutics, announced today the clinical trial of Pyrilutamide as a treatment for the acne vulgaris has completed the first b...
Transcenta Announced Presentation of Preclinical Data of TST005 at 2021 AACR Virtual Annual Meeting
SUZHOU, China, April 15, 2021 /PRNewswire/ -- Transcenta Holding Limited (Transcenta), a clinical stage global biotherapeutics company with fully-integrated capabilities in discovery, development and manufacturing of antibody-based therapeutics, presented preclinical data of TST005, a bi-functio...
Kintor Pharmaceutical Announces AR-PROTAC (GT20029) Approved for Acne and Androgenic Alopecia Clinical Trials in China
SUZHOU, China, April 15, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (stock code 9939.HK, "Kintor Pharmaceutical" or the "Company"), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that it has received approval from t...
Adlai Nortye Announces First Patient Dosed in Global Phase III Clinical Trial of Buparlisib (AN2025) in Combination with Paclitaxel for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma
HANGZHOU, China, April 14, 2021 /PRNewswire/ -- Adlai Nortye, a global biopharmaceutical company focused on developing innovative oncology drugs, today announced that the first patient has been dosed in the global phase III clinical trial (BURAN) at Shanghai Eastern Hospital Medical Center inChin...
Kintor Pharmaceutical and Visum Announced Strategic Partnership to Expand Proxalutamide Manufacturing
SUZHOU, China, April 14, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (stock code 9939.HK, "Kintor Pharmaceutical" or the "Company"), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, recently announced a strategic partnership with Hain...
Molecular Matrix and GenScript Partner to Launch SARS-CoV-2 Neutralizing Antibody Testing Service for Public
PISCATAWAY, New Jersey and WEST SACRAMENTO, Calif., April 13, 2021 /PRNewswire/
--Molecular Matrix, Inc.
Harbour BioMed Presents Novel Antibody for Cancer Immunotherapy at 2021 American Association for Cancer Research Annual Meeting
CAMBRIDGE, Mass., SUZHOU, China and ROTTERDAM, Netherlands, April 13, 2021 /PRNewswire/ -- Harbour BioMed ("HBM"; HKEX: 02142.HK), a global clinical-stage biopharmaceutical company, presents its newly discovered fully human anti-B7H7 monoclonal antibody at the American Association for Cancer Rese...
PharmAbcine presents the non-clinical data of PMC-309 at AACR 2021
DAEJEON, South Korea, April 12, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of antibody therapeutics, announced today that the company presented an e-poster featuring the non-clinical data of PMC-309 at American Associatio...
SkinPen® Precision Now Under Direct Management in the United Kingdom and Ireland
DALLAS, April 12, 2021 /PRNewswire/ -- Crown Aesthetics, a division of Crown Laboratories, Inc., is delighted to announce that SkinPen Precision, the first microneedling device cleared by the FDA and the gold standard for skin remodeling technology, now has a direct presence in the UK andIreland....
Yiling Pharmaceutical doubled its performance through innovation in 2020
SHIJIAZHUANG, China, April 12, 2021 /PRNewswire/ -- Yiling Pharmaceutical (002603.SZ) released its 2020 annual report on Thursday. It achieved an operating revenue ofRMB 8.782 billion in 2020, with a year-on-year growth of 50.76%; and net profit attributed to equity holders ofRMB 1.219 billion, w...
Gracell Biotechnologies Reports Long-term Follow-up Data on TruUCAR-enabled GC027 in Relapsed/Refractory T-ALL at the AACR 2021 Annual Meeting
SUZHOU and SHANGHAI, China, April 10, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, today presented updated long...
Bionomics Successfully Completes A$22.9 million Equity Raise
ADELAIDE, Australia, April 9, 2021 /PRNewswire/ -- Bionomics Limited (ASX: BNO, OTCQB:BNOEF) (Bionomics) has completed its 1 for 6 pro rata non–renounceable entitlement offer that was announced on8 March 2021 (Entitlement Offer) and concurrent placement that was announced on17 March 2021 (Concurr...
Control Bionics (ASX:CBL) Partners with Numotion as Northeastern U.S. Reseller of Assistive Technology Solutions
Strategic Partnership Offers Customers More Access to Control Bionics' Industry-Leading Trilogy Product Line MILFORD, Ohio and NEW SOUTH WALES, Australia, April 9, 2021 /PRNewswire/ -- Control Bionics, a global assistive technology company specializing in speech generating devices, announced it ...
ImmVira Announces Preclinical and Clinical Data to Be Presented at the 2021 ASCO and AACR Annual Meeting
SHENZHEN, China, April 8, 2021 /PRNewswire/ -- ImmVira today announces that the company will be presenting its first innovative product, MVR-T3011, at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting onJune 4-8 2021. Results from the first Phase 1 study of MVR-T3011 as an intr...
Hyundai Bioscience leads in the repurposing of Niclosamide
SEOUL, South Korea, April 8, 2021 /PRNewswire/ -- A research paper by a South Korean bio tech company that demonstrated the drug repurposing of Niclosamide, an anthelmintic known to have excellent therapeutic efficacy against novel coronavirus infection (COVID-19), was published in the world-reno...
Bridge Biotherapeutics Announces Initiation of Phase 1/2 Clinical Trial of BBT-176 in EGFR-Mutant NSCLC with C797S
SEONGNAM, South Korea, April 6, 2021 /PRNewswire/ -- Bridge Biotherapeutics Inc.(288330 KQ), a clinical-stage biotech company headquartered in Seongnam, Republic of Korea, announced that the company has initiated the Phase 1/2 clinical trial assessing safety, tolerability, and anti-tumor activity...
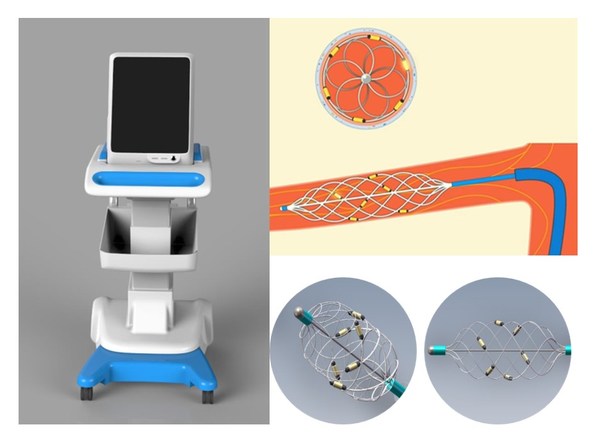
Brattea, a Leading Renal Denervation Company in China, Completes over $US20 Million Series B+ Financing, led by Kuanping Capital
SHANGHAI, April 6, 2021 /PRNewswire/ -- Brattea, a leading renal denervation (RDN) medical technology company inChina, announced today its successful completion of over$US20 million Series B+ financing led by healthcare private equity fund Kuanping Capital. New investors include Hengxu Capital, P...
MitoImmune Received FDA Clearance of IND Application for MIT-001, a Novel Anti-Inflammatory/Anti-Necrotic Therapy for Oral Mucositis in CCRT patients with Head and Neck Cancer
- MitoImmune
US FDA Approved Jemincare to Initiate Clinical Trial of Neutralizing Antibody JMB2002
SHANGHAI, April 2, 2021 /PRNewswire/ -- On March 31, 2021, Jemincare group announced that their anti-SARS-CoV-2 neutralizing antibody, JMB2002, independently developed by Jemincare Shanghai Research Center, was approved by FDA for clinical trial inthe United States. Preclinical data showed that ...
Neurophth Announces IND Approval by the NMPA for Leber Hereditary Optic Neuropathy Gene Therapy
WUHAN and SUZHOU, China, April 1, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd., a fully-integrated genomic medicines company developing adeno-associated viral (AAV)-delivered gene therapies for the treatment of ocular diseases, recently announced the Center for Drug Evaluation (CDE) of China...
Week's Top Stories
Most Reposted
Radisson Hotel Group accelerates growth in Indonesia with ANTA Hotel Bali Canggu, a member of Radisson Individuals
[Picked up by 313 media titles]
2025-12-10 10:00ARKANCE to Launch BIMLOGIQ - A New AI-Powered Extension to Its AEC Tech Stack Offering
[Picked up by 293 media titles]
2025-12-11 09:07Synvo AI and Sobat Bisnis Group (SBG) Forge Strategic Partnership to Bring Secure, Context-Aware Enterprise AI to Indonesia and Southeast Asia
[Picked up by 290 media titles]
2025-12-16 08:00Colebrook Bosson Saunders Chooses India for First Executive Dialogue Exploring Future of Workspaces and Technology
[Picked up by 287 media titles]
2025-12-16 14:26Tiiny AI Reveals World's Smallest Personal AI Supercomputer, Verified by Guinness World Records
[Picked up by 285 media titles]
2025-12-10 16:00