Pharmaceuticals
ACROBiosystems launches GMP-grade DLL4 Protein to accelerate stem cell manufacturing
NEWARK, Del., Nov. 16, 2023 /PRNewswire/ -- ACROBiosystems, a global cornerstone of the pharmaceutical industry committed to offering innovative tools and solutions, recently announces the launch of GMP grade DLL4. GMP grade DLL4 is a recombinant, soluble form of Delta-like Ligand 4, or DLL4, ...
Riding the tide, realize the future: The BD paths for Chinese Biotech
WUXI, China, Nov. 14, 2023 /PRNewswire/ -- 2023 BIO-Europe just closed last week. In line with previous conferences, this BD carnival attracted more than 2,000 companies, 5,000 attendees from close to 60 countries all over the world. Recently, we interviewed a global BD expert, Dr. Kaan Certel. ...
CytoMed Therapeutics to Present at The Benchmark Company's Upcoming Discovery One-on-One Investor Conference
SINGAPORE, Nov. 14, 2023 /PRNewswire/ -- CytoMed Therapeutics Limited
HaemaLogiX Announces Appointment of Damian Clarke-Bruce as Managing Director and CEO
SYDNEY, Nov. 13, 2023 /PRNewswire/ -- HaemaLogiX Ltd, a clinical stage Australian biotech, is pleased to announce the appointment ofDamian Clarke-Bruce as Managing Director and CEO, effective13 November 2023. Outgoing CEO Bryce Carmine will remain active in the company, retaining his role as Cha...
Brii Bio Presents New Data Highlighting Progress Towards Achieving HBV Functional Cure at AASLD's The Liver Meeting® 2023
Direct evidence that BRII-179-induced functional antibody responses can contribute to increased and sustained HBsAg loss rate New insight in utilizing BRII-179 to enriching patients with intrinsic humoral immune responses for higher HBsAg loss or HBV functional cure rates New data support ...
OliPass Discloses Painful but Hilarious Clinical Findings from a Phase 2a Trial in OA Patients with Nav1.7 Selective Inhibitor OLP-1002
SEOUL, South Korea, Nov. 13, 2023 /PRNewswire/ -- OliPass Corporation, an RNA therapeutics platform company, disclosed uncanny clinical findings from a multi-center Phase 2a trial with Nav1.7 selective OLP-1002 in osteoarthritis (OA) patients with moderate to severe pain inAustralia with Novotech...
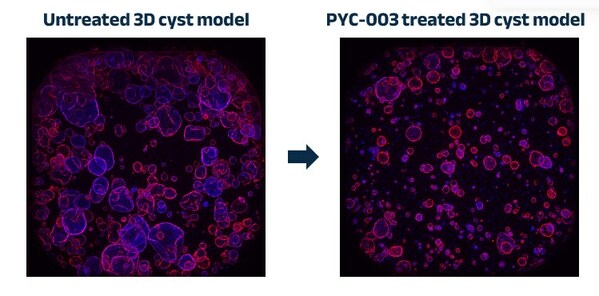
PYC'S FOURTH DRUG CANDIDATE HAS DISEASE-MODIFYING POTENTIAL IN POLYCYSTIC KIDNEY DISEASE
* PYC has developed a new drug candidate for the >5 million people worldwide [1] with Polycystic Kidney Disease (PKD) * This drug candidate has demonstrated efficacy in human models derived from the kidneys of patients with end-stage renal failure due to PKD[2] * PKD is a life-changing diseas...
Nona Biosciences Announces Strategic Collaboration with GeneQuantum Healthcare to Empower Early Discovery of Next-Generation Bioconjugates
CAMBRIDGE, Mass., Nov. 13, 2023 /PRNewswire/ -- Nona Biosciences, a global pioneer of technology innovation and antibody discovery and development solutions, today announced that it has entered into a strategic collaboration with GeneQuantum Healthcare to advance the early discovery of next-gener...
MVR-T3011 IV, Global First Intravenous Oncolytic Product, Successfully Concludes Phase I Clinical Study in the U.S. with Outstanding Safety Results
SHENZHEN, China, Nov. 9, 2023 /PRNewswire/ -- On November 9, 2023, ImmVira announced the successful completion of Phase I clinical study conducted on late-stage patients with various tumor types for our intravenous oncolytic product, MVR-T3011 IV, inthe United States. This milestone was marked by...
PharmAbcine Receives Safety Review Committee Approval to Advance to Dose Level 2 of Clinical Trial of PMC-403 with Neovascular Age-related Macular Degeneration following Dose Level 1 Safety Data
* Safety Review Committee evaluated dose level 1 safety data of PMC-403 and approved advancing to the higher dose treatment. * The starting dose (0.7mg) of PMC-403 showed potential in improving eyesight for neovascular age-related macular degeneration patients with suboptimal response to cur...
Eccogene Enters Exclusive License Agreement With AstraZeneca to Develop and Commercialize Small Molecule GLP-1 Receptor Agonist ECC5004 for Cardiometabolic Diseases
Eccogene will receive initial upfront payment of $185 million. Eccogene is also eligible to receive up to$1.825 billion in future clinical, regulatory, and commercial milestones, as well as royalty payments. AstraZeneca will receive an exclusive license to develop and commercialize ECC5004 in al...
Porton Advanced Announces a Strategic Partnership with BioMap, with AI Models Facilitating AAV Gene Therapy Development
SUZHOU, China, Nov. 8, 2023 /PRNewswire/ -- On November 8, 2023, Porton Advanced announced the establishment of a strategic partnership with BioMap. BioMap will leverage Porton Advanced's unique Adeno-Associated Virus (AAV) vector technology platform and research data to develop AAV assembly effi...
WuXi Biologics Receives AAA MSCI ESG Rating
SHANGHAI, Nov. 7, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), announced it has received an AAA rating from Morgan Stanley Capital International (MSCI) ESG Ratings, the highest rating for compan...
Recruitment target achieved for Phase II SAR-Bombesin prostate cancer trial
SYDNEY, Nov. 7, 2023 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that 50 patients have no...
Kexing Biopharm Attended CPHI Worldwide 2023
SHENZHEN, China, Nov. 7, 2023 /PRNewswire/ -- Recently, CPHI Worldwide 2023 was successfully held inBarcelona, Spain. Kexing Biopharm exhibited more than ten kinds of high-quality medical products in the fields of tumor, autoimmune, anti-virus, etc., including Infliximab, Adalimumab, Bevacizumab,...
Akeso's Cadonilimab (PD-1/CTLA-4) Phase III Trial Meets Primary Endpoint at Interim Analysis Demonstrating Strong Overall Survival Benefit as First-line Treatment in All-comer Patients with Gastric Cancer or Gastroesophageal Junction Adenocarcinoma (GC/GEJC)
HONG KONG, Nov. 7, 2023 /PRNewswire/ -- Akeso (9926.HK) announced positive results from an interim analysis of AK104-302 study, a randomized, double-blind, multicenter, phase III clinical study evaluating PD-1/CTLA-4 bispecific antibody, cadonilimab (开坦尼®) in combination with capecitabine plus...
BioRay Announces First-Patient-In for Phase I Clinical Study of BRY812, a Novel LIV-1 Targeting Antibody Drug Conjugate
SHANGHAI, Nov. 7, 2023 /PRNewswire/ -- BioRay Pharmaceutical Co., Ltd. (hereinafter referred to as BioRay) announced that the first patient has been dosed in the Phase I Clinical trial of BRY812, a third-generation antibody-drug conjugate (ADC) targeting LIV-1 for the treatment of advanced malign...
87.5% ORR | Abbisko presented two clinical updates of Pimicotinib at the 2023 CTOS Annual Meeting
SHANGHAI, Nov. 6, 2023 /PRNewswire/ -- Abbisko Therapeutics Co., Ltd. ("Abbisko Therapeutics" hereafter) announced that two major clinical updates of its CSF-1R inhibitor pimicotinib(ABSK021)were presented at the 2023 Connective Tissue Oncology Society Annual Meeting, which is held inIreland from...
Lerna Bio unveils its first-in-class solution to combat liver failure
SINGAPORE, CAMBRIDGE, Mass. and SHANGHAI, Nov. 6, 2023 /PRNewswire/ -- Lerna Biopharma Pte. Ltd (Lerna Bio) proudly announces the unveiling of its pioneering liver regeneration drug candidate, LR1, at The Liver Meeting inBoston organized by American Association for the Study of Liver Diseases (AA...
I-Mab Announces Participation at Jefferies and Piper Conferences in November
ROCKVILLE, Md. and SHANGHAI, China, Nov. 6, 2023 /PRNewswire/ -- I-Mab (Nasdaq: IMAB) (the "Company"), a global biotechnology company focused on bringing highly differentiated medicines to patients around the world through the discovery, development, and commercialization of novel immunotherapies...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 312 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 307 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00