Pharmaceuticals
Volition appoints Sharon Ballesteros as U.S. Head of Quality and Development Process
AUSTIN, Texas, April 6, 2022 /PRNewswire/ -- VolitionRx Limited (NYSE AMERICAN: VNRX) ("Volition"), a multi-national epigenetics company, has announced the appointment ofSharon Ballesteros as U.S Head of Quality and Development Process. Sharon has over 20 years of experience within the in vitro d...
Kintor Pharma's Proxalutamide Demonstrated Reduction in Hospitalization/Mortality for Patients with Mild to Moderate COVID-19 in Phase III MRCT Study
* Proxalutamide demonstrates a significant reduction in hospitalization/death rate with a protection rate reaching 100% for patients treated more than seven days (p <0.02). * Proxalutamide reduced risk of hospitalization or death especially in those with high-risk factors. * Proxalutamid...
Nihon Medi-Physics Attains World's First Manufacturing of Actinium-225 with Cyclotron on Production Scale for Investigational Drugs
- Core Material Used for Targeted Alpha Therapy (TAT) Expected to Be Novel Treatment for Cancer - TOKYO, April 5, 2022 /PRNewswire/ -- Nihon Medi-Physics Co., Ltd. (NMP), a leading radiopharmaceutical company inJapan, announced on April 5, 2022, that the company has successfully manufactured act...
Indonesia's Ministry of Health Discussed a Universal Vaccine Passport and Verifier for International Travelers
JAKARTA, April 4, 2022 /PRNewswire/ -- Assuming the presidency in the upcoming
G20 summit,Indonesia has begun the Health Working Group (HWG) meeting series
that took place on28-30 March 2022 in Yogyakarta, Indonesia.
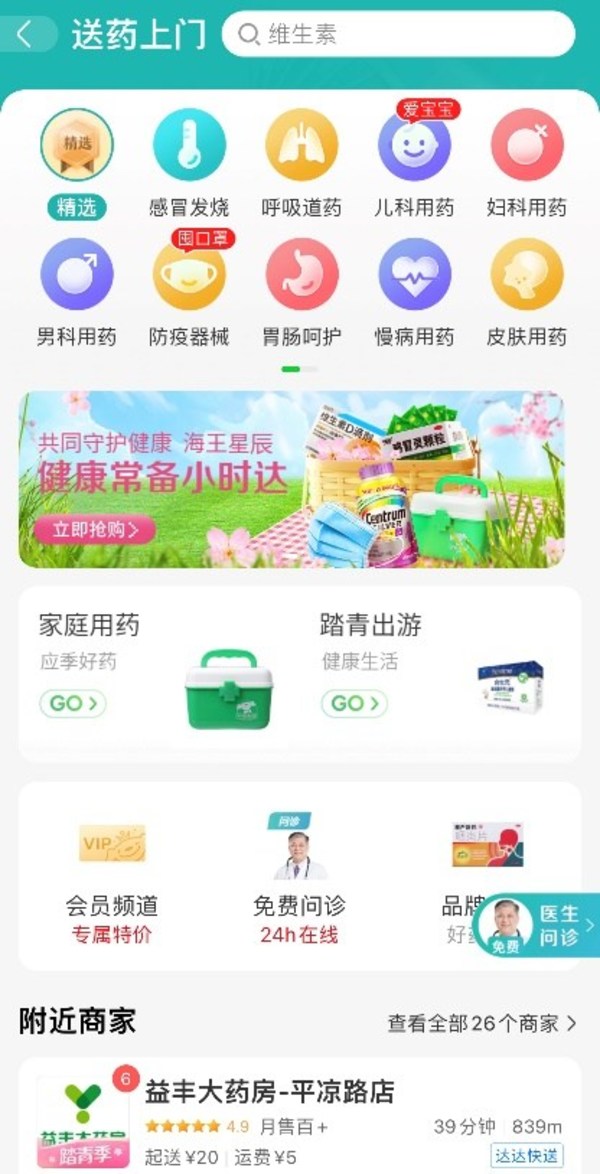
Dada's JDDJ in action to guarantee medical supply and online health consultation with pharmaceutical partners
SHANGHAI, April 2, 2022 /PRNewswire/ -- As a number of cities in China are ramping up anti-epidemic measures, more consumers rely on online-to-offline services to purchase much-needed medications. JDDJ, one ofChina's largest local on-demand retail platforms operated by Dada Group (Nasdaq: DADA), ...
Zhongze Therapeutics Announces Successful Completion Of The First-In-Human Dose Escalation Study of ZZ6398 in Healthy Volunteers
SHANGHAI, April 1, 2022 /PRNewswire/ -- Zhongze Therapeutics, a clinical stage biotech company focused on the discovery, development and commercialization of innovative and transformative therapies for both psychiatric and neurological disorders announced today the successful completion of the fi...
Akeso Reported 2021 Annual Results
Penpulimab Started Commercialization and Cadonilimab Filed for NDA HONG KONG, April 1, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology,...
Zhimeng Biopharma Announces Dosing of First Subject in First-in-Human Phase -- Clinical Trial of CB06
SHANGHAI, March 31, 2022 /PRNewswire/ -- Shanghai Zhimeng Biopharma, Inc. ("Zhimeng"), an innovation-driven biopharmaceutical company focused on the development of innovative drugs for the treatment of chronic hepatitis B and central nervous system diseases, announced dosing of the first particip...
Aceemzyme Co., Ltd., challenging to new drug of brain diseases with recombinase-based ginsenoside Rh2 (s) mass production technology…
- Under development of ginsenoside Rh2(s) mass production technology… - "Enhanced production of ginsenoside Rh2(S) from PPD-type major ginsenosides using BglSk cloned fromSaccharibacillus kuerlensis together with two glycosidase in series" SOUTH KOREA, Seoul, March 31, 2022 /PRNewswire/ -- Ginse...
I-Mab Announces Voluntary Lock-ups by Key Shareholders and Senior Management for Long-Term Commitment
SHANGHAI and GAITHERSBURG, Md., March 31, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the Company's key shareholders including CBC Grou...
LifeTech Scientific Corporation(1302.HK) Announces 2021 Annual Results
Excluding certain non-recurring items, the net profit attributable to owners of the Company increased by 76.8% to RMB 324.0million SHENZHEN, China, March 30, 2022 /PRNewswire/ -- LifeTech Scientific Corporation (LifeTech, 1302.HK), a leading company specialized in minimally invasive interventio...
First Year of Commercialization, Alphamab Oncology Reports Full Year 2021 Financial Results and Business Highlights
SUZHOU, China, March 29, 2022 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) reported financial results for the full year endedDecember 31, 2021 and highlighted recent progress and upcoming milestones. Highlights * First product launch: In November 2021, KN035 (Envafolimab) has receiv...
Biosion licenses BSI-060T (anti-Siglec-15) to Pyxis Oncology
- Pyxis Oncology will be responsible for development and commercialization of BSI-060T (now referred to as PYX-106), a fully human anti-Siglec-15 monoclonal antibody - Biosion earns a $10 million upfront license fee; additional milestone payments and royalties on commercial sales to be earned up...
CellOrigin Closed a New Round of Investment to Jump-start its iPSC Immune Cell Therapy Products Toward Clinics
HANGZHOU, China, March 29, 2022 /PRNewswire/ -- On Mar.21, 2022, CellOrigin Inc, a biotech company focusing on iPSC-derived immune cell therapies, announced it secured a new round of investment of ~100 million RMB from Jifeng Ventures, Kunlun Capital, Yinxinggu Capital and Efung Capital. CellOri...
Laekna Therapeutics Appoints Jeff Porter, Ph.D. as Chairman of Scientific Advisory Board
SHANGHAI and WARREN, N.J., March 29, 2022 /PRNewswire/ -- Laekna Therapeutics, a clinical-stage global biotechnology company,announced today the appointment of Jeff Porter, Ph.D. as the Chairman of its Scientific Advisory Board (SAB). Dr. Porter has over 25 years of global R&D leadership and strat...
New Video Series "PSMA PET/CT TODAY" Discusses the Future of Prostate Cancer Care with Leading Experts
MELBOURNE, Australia and INDIANAPOLIS, March 29, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces the launch of PSMA PET/CT[1] Today, a five-part video series, featuring leading theranostics experts, on the role of advanced diagnostic imaging in pr...
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Year Ended December 31, 2021
* Financial results demonstrate strong fundamentals * Twenty key clinical milestones achieved year-to-date, including positive data readouts for lemzoparlimab, uliledlimab and felzartamab * Seven business development deals, including a US$315M strategic commercial partnership with Jumpcan on...
Samsung Biologics holds 11th Annual General Meeting of Shareholders
INCHEON, South Korea, March 28, 2022 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), the world's leading contract development and manufacturing organization (CDMO), hosted its 11th Annual General Meeting of Shareholders (AGM)today. Five matters were presented and approved unanimously at this ...
Viva Biotech Announced 2021 Annual Results: Revenue Increased by 201.9% YoY, CRO Business Revenue Maintained Rapid Growth with an Increase of 68.7%
Financial Highlights of the year ended December 31, 2021: * Revenue amounted to RMB2,104.1 million, representing a year-on-year (YoY) increase of 201.9% * Gross profit amounted to RMB651.0 million, representing a YoY increase of 113.5% * Adjusted Non-IFRS net profit amounted to RMB352.5 mil...
Clarity's US-based Cu-64 SAR-bisPSMA trial in prostate cancer opens for recruitment
SYDNEY, March 28, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that the diagnostic 64Cu SAR-bisPSMA trial (COBRA NCT05249127 <...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 310 media titles]
2026-02-23 08:00Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 286 media titles]
2026-02-23 10:09Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 280 media titles]
2026-02-17 19:12Yiwu in the Year of the Horse: Understanding the Chinese Expression of a "Global Community"
[Picked up by 260 media titles]
2026-02-19 11:15