Pharmaceuticals
Nippon Express (Nederland) Acquires GDP Compliance Certification for Company-owned Warehouse
TOKYO, Dec. 8, 2021 /PRNewswire/ -- Nippon Express (Nederland) B.V. (hereinafter "NEN"), a local subsidiary of Nippon Express Co., Ltd., obtained Good Distribution Practice (GDP) certification, effectiveSeptember 6, for its company-owned warehouse in Schiphol Trade Park nearAmsterdam's Schiphol ...
China's Largest Commercial GMP Plasmid Manufacturing Facility is Put into Operation, GenScript ProBio Expanding Manufacturing Capacity Again
ZHENJIANG, China, Dec. 7, 2021 /PRNewswire/ -- On December 7th, the "Opening
Ceremony ofGenScript ProBio
I-Mab to Pursue Dual Listing Plan on The Main Board of The Stock Exchange of Hong Kong Limited
SHANGHAI and GAITHERSBURG, Md., Dec. 7, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the Board of Directors of the Company (the "Board") ...
Samsung Biologics adds to global ISO certifications with Quality Management System
* Samsung Biologics earns ISO9001 for Quality Management System, proving its capabilities to ensure high-quality service offerings throughout a product life-cycle * The company is fully certified with the recognition across all of its CDO, CMO and all other core business units in Plant 1,2, a...
Jacobio Receives IND Approval for Combination Therapy of KRAS G12C and Cetuximab Injection in China
BEIJING and SHANGHAI and BOSTON, Dec. 5, 2021 /PRNewswire/ -- Jacobio Pharma ( 1167.HK) has received the Investigational New Drug (the "IND") approval of the combination therapy of KRAS G12C inhibitor JAB-21822 and Cetuximab injection from the Center for Drug Evaluation ofChina (the "CDE") on Dec...
Kazia Announces Positive Final Data From Phase II Clinical Study Of Paxalisib In Newly Diagnosed Glioblastoma
SYDNEY, Dec. 4, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce positive final data from a phase II clinical study of paxalisib as first line therapy in patients with glioblastoma (NCT03522298). The res...
I-Mab Announces First Two Patients Dosed in U.S. Phase 2 Combination Trial of Uliledlimab with Atezolizumab in Patients with Selected Advanced Solid Tumors
SHANGHAI and GAITHERSBURG, Md., Dec. 3, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the first two patients have been dosed in the U.S. p...
Biotheus' Bifunctional Therapeutic PM8001 has been Approved by the USFDA to Enter Phase II Studies
ZHUHAI, China, Dec. 3, 2021 /PRNewswire/ -- Biotheus Inc., has announced that its proprietary anti-PD-L1/TGF-β bifunctional therapeutic, named PM8001, has been approved by the USFDA (United States Food and Drug Administration) for conducting clinical phase II studies as part of an international m...
InnoCare Announces Inclusion of Orelabrutinib in China National Reimbursement Drug List
BEIJING, Dec. 2, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a commercial-stage biotech company, announced today that its BTK inhibitor orelabrutinib has been included in the updated National Reimbursement Drug List (NRDL) by the China National Healthcare Security Administration (NHSA). ...
Hepagene Therapeutics Initiates the RISE Study, a Phase IIa Clinical Trial of HPG1860 in Patients with NASH
SHANGHAI, Dec. 2, 2021 /PRNewswire/ -- Hepagene Therapeutics, Inc, a clinical stage biopharmaceutical company focusing on innovative therapies for patients with liver diseases, today announced that it has screened the first patient in theUSA for the RISE study, a Phase IIa clinical trial of HPG18...
MingMed Biotechnology Announces U.S. FDA Approval for IND Application of HPK1 Small Molecule Inhibitor PRJ1-3024, and Completion of Phase I Clinical Trials for Dry AMD Treatment Drug QA102
GUANGZHOU, China, Dec. 2, 2021 /PRNewswire/ -- MingMed Biotechnology, a clinical stage company dedicated to developing first-in-class pharmaceutical products, announced recently the US Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for PRJ1-3024, a ...
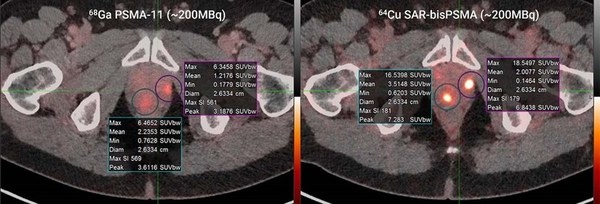
Clarity and Cardinal Health enter into Agreement for Targeted Copper Theranostics
SYDNEY, Dec. 2, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, and Cardinal Health (NYSE: CAH), are pleased to announce that the companies have entered ...
Fifty percent recruitment milestone for PROPELLER prostate cancer trial
SYDNEY, Dec. 1, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that 15 of 30 participants have been recruited in the diagnostic64...
Licensing Partner of Shenzhen Chipscreen Biosciences - HUYABIO International, Receives Regulatory Approval for Chidamide Monotherapy of Peripheral T-Cell Lymphoma(PTCL) in Japan
SHENZHEN, China, Dec. 1, 2021 /PRNewswire/ -- Shenzhen Chipscreen Biosciences' licensing partner, HUYABIO International (HUYABIO™), today announced the regulatory approval for Chidamide (Tucidinostat, also known as Epidaza ®, Hiyasta®, HBI-8000) monotherapy for the treatment of relapsed or refrac...
Binhai New Area to spearhead China's medical industry development
TIANJIN, China, Nov. 30, 2021 /PRNewswire/ -- Here's a news report from chinadaily.com.cn: By 2025, the Binhai New Area in North China's Tianjin municipality will cultivate industry clusters worth tens of billions of yuan that are dedicated to pharmaceuticals, biotech, medical devices, diagnosti...
Ascentage Pharma and Innovent Biologics Announce Approval for China's First Third-Generation BCR-ABL TKI Olverembatinib, Marking a Clinical Breakthrough in the Treatment of Chronic Myeloid Leukemia
SUZHOU, China and ROCKVILLE, Md., Nov. 30, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, and Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-...
Standigm signs MOU with Institut Pasteur Korea for AI-based drug discovery research on infectious disease
SEOUL, South Korea, Nov. 30, 2021 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, today announced the signing of a Memorandum of Understanding ("MOU") with Institut Pasteur Korea ("IPK"), the infectious disease-focused research...
ABM Therapeutics Receives IND Approval in China for BRAF Inhibitor ABM-1310
SAN DIEGO and SHANGHAI, Nov. 30, 2021 /PRNewswire/ -- ABM Therapeutics (ABM), a clinical-stage biopharmaceutical company with a focus on treating brain cancers and cancer metastases, today announced that its IND application for ABM-1310, a new-generation BRAF inhibitor, has been approved by the N...
GBM AGILE Opens to Paxalisib in Canada
SYDNEY, Nov. 29, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an oncology-focused drug development company, is pleased to inform stakeholders that the GBM AGILE study in glioblastoma (NCT03970447) has opened at Sunnybrook Health Sciences Centre inToronto, Ontario. Thi...
OriCiro Genomics Launches New enzymatic DNA synthesis tool for commonly used vectors
TOKYO, Nov. 29, 2021 /PRNewswire/ -- OriCiro Genomics Inc., a pioneer in cell-free synthesis and amplification of genome-scale large DNA for advanced therapeutics and synthetic biology, today announced the launch of OriCiro® Cell-Free Switching system. In the biological sciences, cell-based clon...
Week's Top Stories
Most Reposted
Edufrienz 99: One of Asia's First Digital Platform Advancing Character Education for Future-Ready, Compassionate Children
[Picked up by 319 media titles]
2026-01-23 11:03Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 309 media titles]
2026-01-27 13:00UOB prices AUD2 billion in five-year senior unsecured notes
[Picked up by 307 media titles]
2026-01-23 09:00Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 298 media titles]
2026-01-22 15:16Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 293 media titles]
2026-01-27 18:00