Pharmaceuticals
Nona Biosciences Announces Update on Its Collaborator DualityBio's Antibody-Drug Conjugate (ADC) Collaboration with BeiGene
CAMBRIDGE, Mass., Jan. 3, 2025 /PRNewswire/ -- Nona Biosciences, a global biotechnology company providing a total solution from "Idea to IND" (I to ITM), today announced that BeiGene. Ltd. has exercised its exclusive option to acquire global development, manufacturing, and commercialization right...
Hanx Biopharmaceuticals, Ltd. Announces First Patient Dosing in First-in-Human Phase 1 Clinical Trial of HX044
WUHAN, China, Jan. 2, 2025 /PRNewswire/ -- Hanx Biopharmaceuticals, CO. Ltd, an innovative biotechnology company developing next-generation immunotherapies to address the challenges of unmet medical need diseases, today announced the first patient dosing inAustralia on Dec 30, 2024 for Phase 1 cl...
Fapon Biopharma Introduces FP008 at Biotech Showcase: A Safer and More Effective Immunotherapy for Refractory Cancers
SAN FRANCISCO, Jan. 2, 2025 /PRNewswire/ -- Fapon Biopharma, an innovator in developing therapeutic antibodies and fusion proteins, will present its latest breakthrough immunocytokine, FP008, onJan 14, 2025, at the Biotech Showcase investor conference, following J.P. Morgan Healthcare Week. This ...
FDA Grants Orphan Drug Designation to MicuRx's MRX-5 for NTM Infections
SHANGHAI, Jan. 1, 2025 /PRNewswire/ -- Shanghai MicuRx Pharmaceutical Co., Ltd. ("MicuRx",688373.SH) announced that MRX-5, its self-developed anti-infection drug, has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Administration (FDA) for the treatment ofnon-tuberculous myco...
Ractigen Therapeutics Founder Dr. Long-Cheng Li Awarded 2024 Life Science Ice Breaking Award
NANTONG and SUZHOU, China, Dec. 31, 2024 /PRNewswire/ -- Ractigen Therapeutics proudly announces that its Founder and CEO, Dr.Long-Cheng Li, has been honored with the 2024 Life Science Ice Breaking Award. This award, established by Mr. Cai Lei, a prominent ALS patient and advocate, along with his ...
Duality Biologics Announces B7H4 ADC Milestone Achievement and License Exercise by BeiGene
SOMERSET, N.J., SHANGHAI and SUZHOU, China, Dec. 30, 2024 /PRNewswire/ -- Duality Biologics ("DualityBio") announced that, BeiGene. Ltd. has exercised its exclusive option for the B7H4 antibody-drug conjugate (ADC) DB1312/BG-C9074 from DualityBio, securing global development, manufacturing, and ...
I Peace manufactures 100 lines of GMP iPS Cells cumulatively
Proof of customer recognition of its quality and mass manufacturing capability
PALO ALTO, Calif., Dec. 30, 2024 /PRNewswire/ -- Leading GMP cell CDMO I
Peace, Inc. (https://ipeace.com/en/
Bridge Biotherapeutics to Present at the 43rd Annual J.P. Morgan Healthcare Conference
SEONGNAM, South Korea and BOSTON, Dec. 30, 2024 /PRNewswire/ -- Bridge Biotherapeutics (KQ288330), a clinical-stage biotech company based in South Korea developing novel drugs for fibrosis and cancer, today announced that James Jungkue Lee, Founder and CEO of Bridge Biotherapeutics, will present ...
CARsgen Announces Positive Topline Results from China GC/GEJ Pivotal Phase II Clinical Trial of Claudin18.2 CAR-T (Satri-cel)
SHANGHAI, Dec. 30, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces the positive results from the pivotal Phase II clinical trial CT041-...
Huadong Medicine Co., Ltd. and SynerK reached a strategic cooperation to jointly develop a novel siRNA drug SNK-2726
SUZHOU, China, Dec. 30, 2024 /PRNewswire/ -- Hangzhou Sino-US Huadong Medicine Co., Ltd. (hereinafter referred to as "Huadong Medicine"), a wholly-owned subsidiary of Huadong Medicine Co., Ltd. (SZ.000963), and SynerK PharmaTech (Suzhou) Co., Ltd. (hereinafter referred to as "SynerK") have reache...
Matwings Technology Raised Series A Funding of Tens of Millions USD to Redefine Protein Design
'Beyond Structure, Predicting Function' SHANGHAI, Dec. 25, 2024 /PRNewswire/ -- Recently, Shanghai Matwings Technology Co., Ltd. ('Matwings'), a global leader in AI-driven protein design, announced the successful completion of Series A funding rounds, raising tens of millions of USD, led by Qimi...
Haikou delivers mix of tradition and cutting-edge industry
BEIJING, Dec. 25, 2024 /PRNewswire/ -- A news report from China Daily: Haikou, capital of South China’s Hainan province, is a vibrant metropolis known for its bustling ports, golden beaches and profound culture. Join Michael and Ani on a scavenger hunt in Haikou to explore its unique charm! F...
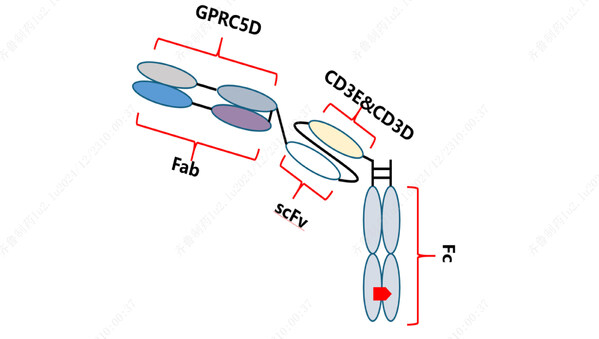
Preliminary Results of Qilu Pharmaceutical's Phase Ia Study on GPRC5D/CD3-Targeting Bispecific Antibody QLS32015 Unveiled at 2024 ASH Annual Meeting
JINAN, China, Dec. 25, 2024 /PRNewswire/ -- During the 66th Annual Meeting of the American Society of Hematology (ASH) held inSan Diego from December 7 to 10 , preliminary results from the first human trial of QLS32015, a novel anticancer drug developed by Qilu Pharmaceutical for treating relapse...
Ractigen Therapeutics Announces First Patient Dosed in Phase I Clinical Trial for RAG-17 in SOD1-ALS
NANTONG and SUZHOU, China, Dec. 24, 2024 /PRNewswire/ -- Ractigen Therapeutics, a clinical-stage pharmaceutical company dedicated to developing RNA-based innovative therapies, today announced the successful dosing of the first patient in the Phase I clinical trial of RAG-17, an innovative siRNA t...
Keymed Biosciences Announces Approval Of Stapokibart For The Treatment Of Chronic Rhinosinusitis With Nasal Polyposis
CHENGDU, China, Dec. 23, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced the National Medical Products Administration (the "NMPA") ofChina has recently approved the supplemental New Drug Application (the "sNDA") of Stapokibart (anti-IL-4Rα monoclonal antibody, trade nam...
Porton Pharma Solutions and Dragon Sail Pharmaceutical Form Strategic Partnership to Build a New Ecosystem for ADC Drug Development, Production, and Supply Chain
CHONGQING, China, Dec. 23, 2024 /PRNewswire/ -- On December 20, 2024, Porton Pharma Solutions (hereinafter referred to as "company" or "Porton") and Dragon Sail Pharmaceutical (Shanghai) Co., Ltd. (a subsidiary of Guilin Sanjin, hereinafter referred to as "Dragon Sail Pharmaceutical") formally si...
Aptamer developed by HKBU for treating rare bone disease 'X-linked hypophosphatemia' receives Orphan Drug Designation and Rare Pediatric Disease Designation by U.S. FDA
HONG KONG, Dec. 23, 2024 /PRNewswire/ -- A research led by Hong Kong Baptist University (HKBU) and the Shanghai Sixth People's Hospital Affiliated to School of Medicine at Shanghai Jiao Tong University (Shanghai Sixth People's Hospital) has discovered that an aptamer developed by HKBU can be used...
CStone Announces Submission of Clinical Trial Application in Australia for CS2009, an Innovative PD-1/VEGF/CTLA-4 Trispecific Antibody
SHANGHAI, Dec. 22, 2024 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), an innovation-driven biopharmaceutical company focused on the research and development of anti-cancer therapies, announced today the submission of clinical trial application inAustralia for CS2009 (PD-1/VEGF/CT...
YolTech Therapeutics Announces Successful Completion of Dose Escalation Phase in Phase I Trial of YOLT-201 for ATTR
SHANGHAI, Dec. 21, 2024 /PRNewswire/ -- YolTech Therapeutics today announced updated data from its ongoing Phase I/IIa clinical trial of YOLT-201, a first-in-class CRISPR/Cas9-based in vivo gene-editing therapy for ATTR amyloidosis. The trial has completed dosing in eight participants, including ...
United Imaging Intelligence at RSNA 2024: Empowering a More Intelligent and Connected World with Medical AI
CHICAGO, Dec. 20, 2024 /PRNewswire/ -- As RSNA 2024 concluded, United Imaging Intelligence (UII), a subsidiary of United Imaging Group specializing in medical AI, made a significant impression with a visionary showcase that seamlessly aligned with the event's theme: "Building Intelligent Connecti...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 328 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 310 media titles]
2026-02-26 19:57HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 309 media titles]
2026-02-26 09:30Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13