Health
Sanyou Bio Congratulates KangaBio on their IND Approval for Next-Generation IL-12 Prodrug Cancer Immunotherapy
SHANGHAI, Nov. 4, 2023 /PRNewswire/ -- On October 26, 2023, KangaBio announced that the U.S. FDA has granted official approval for their independent R&D clinical trial application (IND) for KGX101. KGX101 is a recombinant IL-12 Fc fusion protein designed for intravenous injection. The KGX101 clin...
The 2nd Boao International Conference on Real World Studies of Medical Products Held in Hainan
HAIKOU, China, Nov. 3, 2023 /PRNewswire/ -- On 31st Oct., the 2nd Boao International Conference on Real World Studies of Medical Products, held in Boao,Hainan, was attended by nearly 1,000 participants from domestic and international pharmaceutical regulatory agencies, academic societies, indust...
ASH 2023 | Ascentage Pharma to Present Results from Three Clinical Studies of Bcl-2 Inhibitor Lisaftoclax (APG-2575), Including the First Data in AML and MM
SUZHOU, China, and ROCKVILLE, Md., Nov. 2, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that results from three clinical studies of lisaft...
ASH 2023 | For the Sixth Consecutive Year, Results from Multiple Clinical Studies of Olverembatinib Have Been Selected for Presentations, Including Two Oral Reports, at the 2023 ASH Annual Meeting
SUZHOU, China, and ROCKVILLE, Md., Nov. 2, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that results from multiple clinical studies of the ...
PolyActiva reports promising clinical trial results for its 6-month sustained drug delivery, biodegradable ocular implant in glaucoma patients.
* Melbourne ophthalmology company PolyActiva is developing a unique biodegradable ocular implant that provides 6 months of sustained drug delivery for patients with glaucoma, the second most common cause of irreversible blindness. * The interim Phase 2a results demonstrate a sustained >20% re...
Complete Genomics and Basepair™ Announce Technology Integration Partnership
SAN JOSE, Calif. and NEW YORK, Nov. 2, 2023 /PRNewswire/ -- Complete Genomics and Basepair, Inc today announced a technology integration partnership to accelerate the analysis and interpretation of genomic data generated by Complete Genomics' DNBSEQTM sequencing platforms*. The partnership gives ...
Ivonescimab's Novel Mechanism of Action Highlighting Cooperative Binding to be Featured in Poster Presentation at SITC 2023
Potential First-in-Class Tetravalent Bispecific Antibody Demonstrates Enhanced Binding inthe Simultaneous Presence of PD-1 & VEGF Cooperative Binding of Ivonescimab Enables Higher Avidity in the Tumor Microenvironmentwith Over 18 Fold Increased Binding Affinity to PD-1 in the Presence of VEGF in...
Felzartamab Granted Breakthrough Therapy Designation by U.S. Food and Drug Administration (FDA) for Primary Membranous Nephropathy (PMN)
* FDA granted Breakthrough Therapy Designation for felzartamab in PMN upon positive clinical data from M-PLACE, a Phase 2 study led by I-Mab partner HI-Bio * I-Mab has full development and commercialization rights of felzartamab in Greater China for all indications, with Phase 3 multiple myelom...
Boryung Successfully Concludes 'Humans In Space Symposium' at 2023 ASCEND in Las Vegas
* HIS Symposium co-located with ASCEND, the world's event designed to accelerate the building of our sustainable off-world future, fromOctober 23 to 25. * Jay Kim, the Chairman and CEO of Boryung, invited as a speaker for ASCEND's opening and closing ceremonies and the panel discussion. * B...
Chipscreen's New Drug Candidate CS32582 Capsules Approved for Clinical Treatment of Psoriasis
SHENZHEN, China, Nov. 2, 2023 /PRNewswire/ -- On Oct. 31, 2023, Shenzhen Chipscreen Biosciences Co., Ltd. (hereinafter referred to as "Chipscreen" and with stock code 688321.SH) received the "Drug Clinical Trial Approval Notice" issued by the National Medical Products Administration ("NMPA") thro...
Precision, Effectiveness, and Intelligence: Explore the Latest Trend of Healthcare and Medical Equipment Industry at 134th Canton Fair
GUANGZHOU, China, Nov. 1, 2023 /PRNewswire/ -- As the public is paying increasing attention to health management, the 5-day onsite exhibition of Phase 3 of the 134th Canton Fair held from October 31st to November 4th will present nearly 600 quality exhibitors in medical and healthcare industries ...
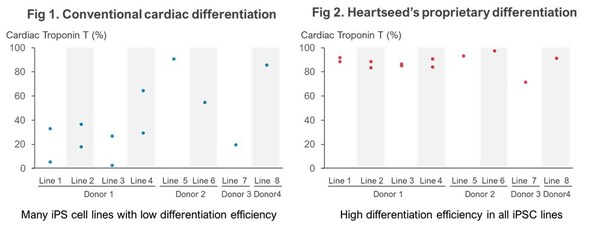
Using iPS Cells from I Peace, Heartseed Succeeds in Stable Production of High Purity Cardiomyocytes, A Major Step Forward in Advancing Autologous Cardiac Regenerative Medicine
PALO ALTO, Calif., Nov. 1, 2023 /PRNewswire/ -- Leading GMP cell CDMO I Peace,
Inc. (https://www.ipeace.com
AI-Powered Tumor Microenvironment Analysis Predicts Treatment Outcomes in NSCLC Patients with EGFR Mutation: Groundbreaking Studies to be Presented by Lunit at SITC 2023
* Six posters featuring the Lunit SCOPE suite and new insights into the tumor microenvironment are to be showcased at the SITC 2023 Annual Meeting SEOUL, South Korea, Nov. 1, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therap...
Jolly Good Inc. Establishes North American Subsidiary: Joint Development of Medical VR for "Apple Vision Pro" with U.S. Experts
~ First Phase: Joint Development of Chronic Pain VR with U.S. Chronic Pain Experts TOKYO, Nov. 1, 2023 /PRNewswire/ -- Jolly Good Inc. (Chuo-ku, Tokyo, CEO: Kensuke Joji, hereinafter referred to as Jolly Good), which develops and provides medical VR, announces that it has established a North Ame...
I-Mab Announces Poster Presentations of 4-1BB Bispecific Antibody Portfolio at SITC 2023
ROCKVILLE, MD, U.S. and SHANGHAI, China, Nov. 1, 2023 /PRNewswire/ -- I-Mab (Nasdaq: IMAB) (the "Company"), a global biotechnology company focused on bringing highly differentiated medicines to patients around the world through the discovery, development, and commercialization of novel immunother...
Curocell Completes Korea's First Phase 2 Clinical Trial for Next-Generation CAR-T
* Anbal-cel targeting DLBCL completes phase 2 trial * Anticipation growing over Korea's first CAR-T therapy ahead of regulatory review * Commercial manufacturing scheduled in 2025 at Korea's only CAR-T GMP facility DAEJEON, South Korea, Nov. 1, 2023 /PRNewswire/ -- Curocell, South Korea ...
Complete Genomics demonstrates technical and commercial momentum in the sequencing market through new customers, partnerships and collaborations less than one year after launching in the U.S.
WASHINGTON, Oct. 31, 2023 /PRNewswire/ -- Complete Genomics, a pioneering genomic sequencing company, announced today at the American Society of Human Genetics (ASHG) Annual Meeting,Nov. 1-5, in Washington details on the commercial and technical momentum it has demonstrated in the last 10 months ...
Lunit Joins as an Associate Partner with the World Economic Forum
- Lunit joins as an Associate Partner of the World Economic Forum, propelling global expansion and collaboration in the realm of AI-driven cancer care SEOUL, South Korea, Oct. 31, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and th...
Meihua International Medical Technologies Co., Ltd. Establishes A Subsidiary Dedicated to Introducing International Patented Pharmaceuticals and Medical Device Technologies to China and Hainan Free Trade Port Boao Hope City
YANGZHOU, China, Oct. 31, 2023 /PRNewswire/ -- Meihua International Medical Technologies Co., Ltd. ("MHUA" or the "Company") (NASDAQ: MHUA), a reputable manufacturer and provider of Class I, II and III disposable medical devices with operating subsidiaries inChina, announced today the establishme...
CHIMERIC THERAPEUTICS ANNOUNCES FDA CLEARANCE OF IND APPLICATION FOR CHM 2101, A NOVEL CDH17 CAR T CELL THERAPY FOR ADVANCED GASTROINTESTINAL CANCERS
* FDA IND Clearance for CHM 2101, a novel 3rd generation CDH17 CAR T cell therapy * Anticipated to be the first CDH17 CAR T cell therapy to enter the clinic * Phase 1A clinical trial to initiate patient enrolment in 2024 * Phase 1A clinical trial will enroll patients with advanced Colorecta...
Week's Top Stories
Most Reposted
Mastercard goes OTP-free in APAC for faster, safer online transactions
[Picked up by 326 media titles]
2024-11-06 09:00Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 309 media titles]
2024-11-12 09:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
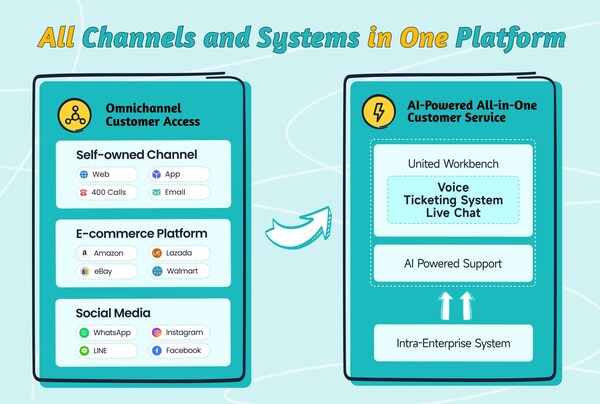
2024-11-11 21:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 284 media titles]
2024-11-12 11:00