Health
Lunit Makes Full-Fledged Entry into the Middle East with Participation in 'Saudi Vision 2030' Healthcare Transformation Project
* Lunit's AI-powered cancer screening solutions have been selected for Seha Virtual Hospital's AI evaluation and clinical integration project * Successful validation to be followed by supply contract to assist Saudi Arabia's national cancer and tuberculosis screening program SEOUL, South Korea...
I-Mab Announces Publication of Claudin18.2 x 4-1BB Bispecific Antibody Givastomig in JITC
GAITHERSBURG, Md. and SHANGHAI, July 5, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of pioneering immunotherapies, today announced the publication of a manuscript entitled "C...
TiumBio and Hansoh Pharma Announce Clinical Trial Approval of 'HS-10518/TU2670' from NMPA in China
- Hansoh plans to develop 'TU2670 (Hansoh's code: HS-10518)' as a best-in-class novel GnRH antagonist inChina - TiumBio will complete the last patient dosing in a Phase 2a clinical trial of TU2670 in endometriosis inEurope by the end of the year BOSTON and SEONGNAM, South Korea, July 5, 2023 /P...
BGI CEO Yin Ye Advocates for Global Technology Sharing
SHENZHEN, China, July 5, 2023 /PRNewswire/ -- Speaking on a panel at the 10th Arab-China Business Conference inRiyadh, Saudi Arabia, Dr Yin Ye, BGI Group CEO, reinforced the importance of making technological advancements available to everyone through scientific sharing to benefit humanity. "We ...
Jacobio Receives CDE Approval for Glecirasib's Pancreatic Cancer Pivotal Study in China
BEIJING, SHANGHAI and BOSTON, July 4, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced its novel KRAS G12C inhibitor glecirasib's pivotal study for pancreatic cancer has been approved from CDE (Center for Drug Evalu...
Positive guidance from US FDA on Cu-64 SAR-bisPSMA Phase III trial in prostate cancer
Highlights * FDA agreement for a pivotal Phase III trial for 64Cu SAR-bisPSMA diagnostic in prostate cancer * Phase III trial design based on 64Cu SAR-bisPSMA data package, including positive results from the completed PROPELLER trial * A total of 383 prostate cancer patients to take part ...
China NMPA approves RareStone's pitolisant (Wakix) for the treatment of narcolepsy
-- pitolisant is the first and only treatment drug approved in mainland China for narcolepsy. SHANGHAI, July 4, 2023 /PRNewswire/ -- RareStone Group, a rare disease-focused company aiming to establish the first rare disease ecosystem in China, announced that on June 30, the Chinese National Med...
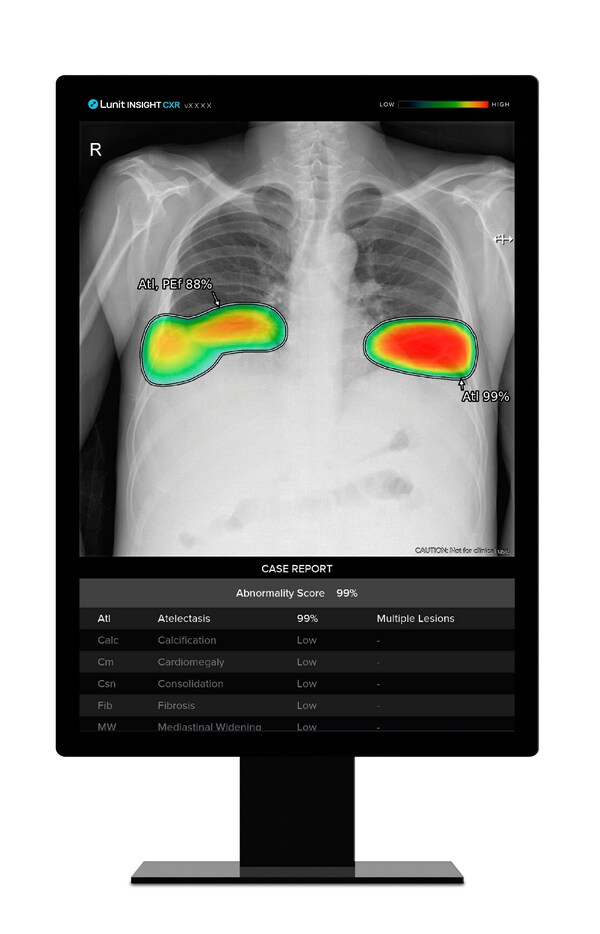
Lunit's AI-Powered Lung Cancer Screening Solution Significantly Affects Radiologists' Diagnostic Determination - Published in Radiology
- Recent study conducted by Seoul National University Hospital provides strong evidence that high-accuracy AI model improves radiologists' chest X-ray analysis performance SEOUL, South Korea, July 3, 2023 /PRNewswire/ -- Findings from a recent study demonstrate that medical AI solutions with onl...
Skyline Therapeutics Receives FDA Clearance of IND for SKG0106, a Novel Intravitreally Delivered AAV Gene Therapy Candidate for Neovascular Age-related Macular Degeneration
SHANGHAI and BOSTON, July 3, 2023 /PRNewswire/ -- Skyline Therapeutics, an innovation-driven gene therapy company committed to developing unique and novel solutions for rare and severe diseases, today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New D...
Henlius Forecasts Profit in 1H 2023
Spurring a Seamless R&D, Manufacturing, and Commercialisation Positive Cycle SHANGHAI, July 3, 2023 /PRNewswire/ -- Henlius (2696.HK) released a positive profit forecast. Based on the preliminary assessment of the unaudited comprehensive management accounts for the six months endedJune 30, 2023, ...
Zymedi announced their collaboration with the National Heart, Lung, and Blood Institute through a CTA CRADA to develop ZMA001 mAb, a potential treatment for Pulmonary Arterial Hypertension (PAH), a rare, female predominant disease
SEOUL, South Korea, July 2, 2023 /PRNewswire/ -- Today Zymedi, a Korean biotech venture-backed company, announced the signing of a clinical Cooperative Research and Development Agreement (CRADA) with the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health ...
Chime Biologics Announces Strategic Cooperation with Leads Biolabs and BeiGene to Advance LBL-007 mAb Development and Manufacturing Globally
* Leads Biolabs and Chime Biologics initiate strategic cooperation to accelerate IND application and provide clinical trial materials inChina. * BeiGene and Chime Biologics establish strategic cooperation to facilitate IND applications overseas and clinical trial materials supply. SHANGHAI, Ju...
Harbour BioMed Announces Biologics License Application Acceptance of Batoclimab for Treatment of Generalized Myasthenia Gravis by NMPA
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, June 29, 2023 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
Jowell Global Ltd. Granted Extension to Meet Nasdaq Minimum Bid Price Requirement
SHANGHAI, June 29, 2023 /PRNewswire/ -- Jowell Global Ltd. ("Jowell Global" or the "Company") (NASDAQ: JWEL), one of the leading cosmetics, health and nutritional supplements, and household products e-commerce platforms inChina, today announced that, onJune 27, 2023, the Company received a writte...
HaemaLogiX Announces Positive Final Results from KappaMab Combination Phase IIb Myeloma Trial
* Significant improvement in Overall Response Rate (83%) compared with matched case control group (45%). * Significant overall survival advantage, with a 46% reduction in the risk of death. * Excellent safety profile and significant efficacy bolster HaemaLogiX's plan to progress further Kap...
Jacobio Pharma Presents Clinical Results of Glecirasib in Colorectal Cancer
BEIJING, KYOTO and BOSTON, June 29, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced clinical results of its novel KRAS G12C inhibitor glecirasib monotherapy and in combination therapy with cetuximab to treat KRAS G...
Clarity establishes a US Center of Excellence for Targeted Copper Theranostics
SYDNEY, June 28, 2023 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the establishment of a ...
Flat Medical Expands Leadership Team to Manage Growth in Europe and the United States
TAIPEI, June 28, 2023 /PRNewswire/ -- Flat Medical Co. Ltd., a leading MedTech company specializing in safety solutions for anesthesia and critical care, is pleased to announce the appointment of two executives who will spearhead the company's expansion inEurope and America. Jan Wormmeester has b...
Sanyou Forms Partnership with Hangzhou Zhongmei Huadong Pharmaceutical, Catalyzing Innovative Drug Research and Development
SHANGHAI, June 28, 2023 /PRNewswire/ -- Recently, Sanyou Biopharmaceuticals Co., Ltd. (hereinafter referred to as Sanyou) and Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd, a wholly-owned subsidiary of Huadong Medicine Co., Ltd. (SZ.000963) (hereinafter referred to asHuadong Medicine), signed...
Caliway Announces Positive Data from CBL-0201EFP Phase 2-Stage 1 Study of CBL-514 in Treating Moderate to Severe Cellulite
* CBL-514 is the only non-invasive product that can improve 1-level of cellulite severity two weeks after a single treatment. * The highest dose of CBL-514 treatment demonstrated the best efficacy, 87.5% of thighs achieved at least 1-level improvement in cellulite severity two weeks after CBL...
Week's Top Stories
Most Reposted
Mastercard goes OTP-free in APAC for faster, safer online transactions
[Picked up by 326 media titles]
2024-11-06 09:00Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 309 media titles]
2024-11-12 09:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
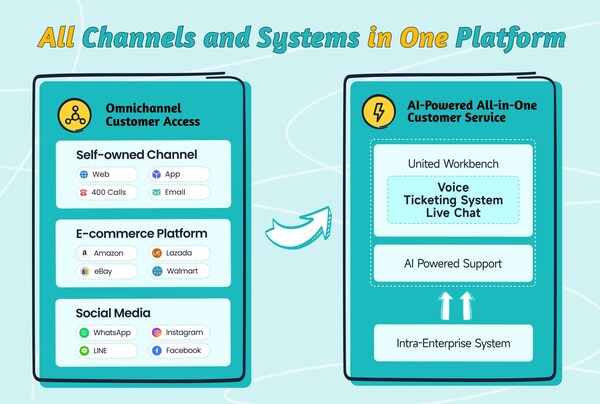
2024-11-11 21:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 284 media titles]
2024-11-12 11:00