Health
Keymed Biosciences Announces Dosing of First Patient in Phase I Trial of Bispecific Antibody CM350
CHENGDU, China, June 10, 2022 /PRNewswire/ -- Keymed Biosciences (HKEX: 02162) announced today that the first patient has been dosed in the Phase I trial of CM350. CM350 is a GPC3xCD3 bispecific antibody developed by the Company for the treatment of solid tumors. The phase I trial is being conduc...
J INTS BIO successfully held two Korean Advisory Board Meetings for its Novel Oral 4th Generation EGFR TKI (JIN-A02)
Members include the 10 leading NSCLC experts in Korea, discussing clinical design and R&D… SEOUL, South Korea, June 10, 2022 /PRNewswire/ -- J INTS BIO announced that it had conducted two Korean Advisory Board Meetings (ABM), first inDecember 2021 and the second in April this year for its novel ...
RAD adds brain tumor technology to portfolio
* Sublicensing of promising imaging & therapeutic radiopharmaceutical from leading US university,Case Western Reserve University (CWRU), Ohio * PTPµ (PTPmu), the target, is a unique biomarker present only in tumor cells but not healthy cells * The radionuclide carrying PTPµ-targeting agent h...
Inteleos Announces Maya Health Alliance as MissionPOCUS Selection for 2022
ROCKVILLE, Md., June 9, 2022 /PRNewswire/ -- Today Inteleos announced a pilot project withWuqu' Kawoq | the Maya Health Alliance as the selection of the Point-of-Care Ultrasound (POCUS) Certification Academy's 2022 MissionPOCUS™ project. As the fifth MissionPOCUS project since the initiative's in...
BIORCHESTRA, presenting lead candidate BMD-001 for Alzheimer's disease and drug delivery system technology at the 2022 BIO International Convention
BOSTON, June 9, 2022 /PRNewswire/ -- BIORCHESTRA Co., Ltd (BIORCHESTRA), is scheduled to give a company presentation onJune 16, 12:00 at BIO International convention, taking place inSan Diego, California, between June 13-16, 2022. BIORCEHSTRA's company presentation will cover its lead therapeut...
Health Canal Appoints New Formal Owner In Efforts To Become Global Leader In Medical Health News
GROVE CITY, Ohio, June 9, 2022 /PRNewswire/ -- Health Canal, the fastest growing online health news publication, announced the appointment ofErik Pham as the new formal owner - this is in support of the company's new objectives to provide health and medical content publishing to untapped audience...
Standigm Signs MOU with Merck Korea for AI drug Discovery Research
- Merck's retrosynthesis AI software 'SYNTHIA™ retrosynthesis software' accelerates Standigm's synthesis capability SEOUL, South Korea, June 9, 2022 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, today announced the signing o...
Amaroq Therapeutics hits early milestones and makes key appointments to Scientific Advisory Board
Promising 'dark matter' cancer therapy has clinical trials in sight DUNEDIN, New Zealand, June 8, 2022 /PRNewswire/ -- Amaroq Therapeutics, a biotechnology company focussed on developing a new class of therapeutics that target lncRNA in cancer, is progressing towards clinical trials following a ...
OnCusp Therapeutics and Multitude Therapeutics Enter into an Ex-China Licensing Agreement for a Potentially Highly Differentiated CDH6-Targeting Antibody Drug Conjugate
NEW YORK, June 8, 2022 /PRNewswire/ -- OnCusp Therapeutics announced today a licensing agreement with Multitude Therapeutics for the development and commercialization of AMT-707 (now referred to as CUSP06), a potentially highly differentiated second-in-class CDH6 antibody drug conjugate ("ADC"). ...
Harbour BioMed Announces IND Approval for B7H4x4-1BB Bispecific Antibody
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, June 8, 2022 /PRNewswire/ --Harbour BioMed ("HBM", HKEX: 02142) announced that China National Medical Products Administration (NMPA) had approved the investigational new drug (IND) application to commence phase I trial of its B7H4x4-...
Waterdrop Inc. to Report First Quarter 2022 Financial Results on June 15, 2022
BEIJING, June 8, 2022 /PRNewswire/ -- Waterdrop Inc. (NYSE: WDH) ("Waterdrop" or the "Company"), a leading technology platform dedicated to insurance and healthcare service with a positive social impact, today announced that it will report its unaudited financial results for the first quarter end...
ASCO 2022 | Ascentage Pharma Releases Updated Data Demonstrating Lisaftoclax's (APG-2575) Therapeutic Potential in Patients with R/R CLL/SLL
SUZHOU, China and ROCKVILLE, Md., June 7, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the updated results from a Pha...
ASCO 2022 | Ascentage Pharma Releases for the First Time Results of its FAK/ALK/ROS1 inhibitor APG-2449 Demonstrating Safety and Efficacy in Patients with Advanced NSCLC
SUZHOU, China, and ROCKVILLE, MD, June 7, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the results from its Phase I ...
Snap Fitness Announces "Earn Your Apple Watch" Program
New Incentive Program Rewards Active Members while Benefiting Franchisees with Brand Differentiation CHANHASSEN, Minn., June 8, 2022 /PRNewswire/ -- In a strategic move that further sets them apart as a leader in the fitness space, Snap Fitness announced today the global launch of the "Earn Y...
The 3rd BIO International Convention 2022 is Approaching
SAN DIEGO, June 7, 2022 /PRNewswire/ -- With the 3rd BIO International Convention 2022 soon approaching, Neurophth Therapeutics is looking forward to discussing collaboration opportunities in gene therapy for both rare and common ocular diseases. Neurophth Therapeutics is a leading clinical-stag...
PHASE II STUDY OF PAXALISIB IN BRAIN METASTASES ADVANCES TO EXPANSION STAGE IN BREAST CANCER BRAIN METASTASES COHORT
SYDNEY, June 7, 2022 Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that a phase II, genomically-guided study of multiple therapies in patients with brain metastases, led by the Alliance for Clinical Trials in Oncology (NC...
Chordia Therapeutics Announces Interim Results of the Phase 1 Clinical Trial of CLK Inhibitor CTX-712 at the 2022 ASCO Annual Meeting
KANAGAWA, Japan, June 7, 2022 /PRNewswire/ -- Chordia Therapeutics Inc. ("Chordia"), a biotech company engaged in the research and development of novel therapies for cancers, today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712, a selective pan-CDC-...
ASCO 2022 | Ascentage Pharma Releases Updated Results Demonstrating the Therapeutic Potential of Alrizomadlin (APG-115) plus Pembrolizumab in Patients with Solid Tumors who Progressed on Immunotherapies
SUZHOU, China, and ROCKVILLE, Md., June 6, 2022 /PRNewswire/ -- scentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the updated results from a Pha...
ASCO 2022 | The First Dataset of Olverembatinib (HQP1351) in Patients with GIST Demonstrates Therapeutic Potential with a Clinical Benefit Rate of 83.3%
SUZHOU, China, and ROCKVILLE, Md., June 6, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the latest results from a Pha...
iNtRON Completes GLP-TOX Studies of BAL200
* A novel drug candidate for anthrax received orphan drug designation (ODD) by the US FDA. BOSTON and SEOUL, Korea, June 6, 2022 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON" or "Company") announced today that the company has successfully completed the GLP toxicology studies of BAL200. The co...
Week's Top Stories
Most Reposted
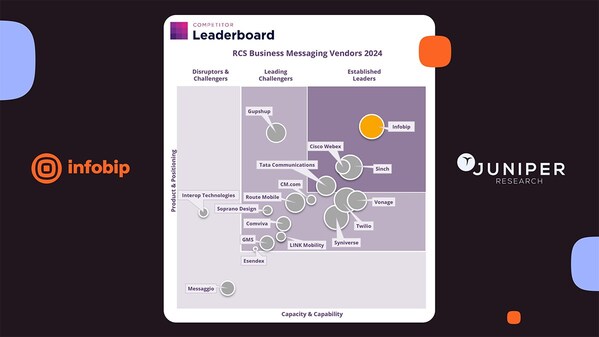
Infobip recognized as RCS Business Messaging Leader by Juniper Research
[Picked up by 331 media titles]
2024-11-05 18:06Mastercard goes OTP-free in APAC for faster, safer online transactions
[Picked up by 326 media titles]
2024-11-06 09:00Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Mastercard launches Pay Local, enabling Asia's digital wallet providers to process card payments from more than 2 billion Mastercard cardholders
[Picked up by 317 media titles]
2024-11-05 09:00XtalPi and Sinar Mas Multiartha Launch Strategic Partnership to Revolutionize AI Across Asia-Pacific
[Picked up by 277 media titles]
2024-11-07 11:34