Health
China.org.cn: Hong Kong's fight against COVID-19: a test for "one country, two systems"
BEIJING, March 4, 2022 /PRNewswire/ -- A news report from China.org.cn on Hong Kong's COVID-19 pandemic situation:   Hong Kong has been taken aback by the 5th onset of the COVID-19 pandemic, and it's spreading fast.Hong Kong is a metropolis that runs under the "one country, two...
Aravax Pty Ltd announces opening of IND for Phase 2 clinical trials of PVX108, a next-generation immunotherapy for the treatment of peanut allergy
* Phase 2 study of PVX108 to commence in United States and Australia * Dr Robert A. Wood, professor of pediatrics at the Johns Hopkins University School of Medicine, joins Scientific Advisory Board MELBOURNE, Australia, March 4, 2022 /PRNewswire/ -- Aravax, a clinical stage biotechnology compa...
111, Inc. Reaches Strategic Partnership on Direct Supply with Beilin Pharmaceutical to Blaze New Trails in Digital Development of Chinese Patent Medicine
SHANGHAI, March 4, 2022 /PRNewswire/ -- On February 23, 2022, 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company inChina, signed a strategic cooperation agreement on direct supply with Xi'an Beilin Pharmaceutical Co., Ltd. ("Beilin Pharmaceutical")...
Genome and Company announces Clinical Trial Collaboration with MSD to evaluate 'GEN-001' in combination of KEYTRUDA® (pembrolizumab) in phase 2 clinical trial in biliary tract cancer patients
SEOUL, South Korea, March 3, 2022 /PRNewswire/ -- Genome and Company (KOSDAQ: 314130, CEO∙CTO: Jisoo Pae∙Hansoo Park), a leading global microbiome anti-cancer drug development company, announced it has entered into a first Clinical Trial Collaboration and Supply Agreement (CTCSA) with MSD (a trad...
InxMed Raised $50 million in Series B Financing to Advance Innovative Therapies to Drug-resistance Cancers
NANJING, China, March 3, 2022 /PRNewswire/ -- InxMed Co., Ltd, a clinical-stage biotechnology company dedicated to developing innovative therapies targeting stroma microenvironment and drug resistance for hard-to-treat solid tumors, today announced that it had completed $50 million in Series B Fi...
Claudin18.2 CAR T Cells (CT041) Receives Approval to Initiate A Confirmatory Phase II Clinical Trial for advanced GC/GEJ in China
SHANGHAI, March 3, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T cell therapies for the treatment of hematologic malignancies and solid tumors, today announced that the Center for Drug Evaluation (CDE) of the National Medic...
I-Mab Receives FDA Orphan Drug Designation for its Novel Claudin 18.2 x 4-1BB Bispecific Antibody TJ-CD4B for the Treatment of Gastric Cancer
SHANGHAI and GAITHERSBURG, Md., March 3, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the U.S. Food and Drug Administration (FDA) has gr...
Akeso Announces Clinical Trial Collaboration with Chipscreen Biosciences to Evaluate Cadonilimab in Combination with Chiauranib for Extensive-Stage Small-Cell Lung Cancer
HONG KONG, March 2, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ("Akeso"), a biopharmaceutical company dedicated to the research, development, manufacturing and commercialization of innovative antibody drugs that are affordable to patients worldwidetoday announced that it has entered into a collab...
Seegene appoints Richard Creager as CEO of U.S. subsidiary to bolster U.S. business
SEOUL, South Korea, March 2, 2022 /PRNewswire/ -- Seegene Inc.
REDCLOUD BIO ANNOUNCES FDA ACCEPTANCE OF IND APPLICATION FOR NEXT-GENERATION EGFR INHIBITOR H002 IN NON-SMALL CELL LUNG CANCER
* Drug Candidate Is First From Company To Enter Phase I/IIa Clinical Trial * H002 Has Potential To Overcome Resistance Driven By Various EGFR C797S-Containing Mutations in Non-Small Cell Lung Cancer SHANGHAI, March 2, 2022 /PRNewswire/ -- RedCloud Bio (the "Company"), an innovative biotech com...
InnoCare Announces Approval of Phase II Clinical Trial Using Orelabrutinib for the Treatment of NMOSD in China
BEIJING, March 1, 2022 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a leading biopharmaceutical company, announced today the Investigational New Drug (IND) approval of its Bruton's tyrosine kinase (BTK) inhibitor orelabrutinib by China National Medical Products Administration (NMPA) to start ph...
WuXi Biologics Wins CMO Leadership Awards in All Six Core Categories for Fifth Consecutive Year
SHANGHAI, March 1, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global CRDMO service company, today announced that the company has won the 2022 CMO Leadership Awards in all six core categories (i.e., capabilities, compatibility, expertise, reliability, quality and service).2022 m...
ImmVira's breakthrough intravenous oncolytic virus product MVR-T3011 IV completed first dosing in Phase I clinical trial in China
SHENZHEN, China, March 1, 2022 /PRNewswire/ -- Following its robust safety data in the U.S. Phase I clinical study, ImmVira announced that its global first intravenous administered oncolytic herpes simplex virus ("oHSV") product MVR-T3011 IV has completed first dosing onMarch 1, 2022 and initiate...
Vocera, now part of Stryker, introduces latest evolution in hands-free communication to help protect and connect healthcare workers
New Minibadge expands intuitive mobile solutions for care team collaboration and safety KALAMAZOO, Michigan, USA, March 1, 2022 /PRNewswire/ -- Vocera, now part of Stryker, a global leading medical technology company, introduced the new Minibadge, a wearable, voice-driven device that enables mobi...
Kintor Pharma Announces First Patient Dosing in Phase II Clinical Trial of KX-826 for Treatment of Androgenic Alopecia Patients in the US
SUZHOU, China, March 1, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma," HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced the first patient dosing in its phase II clinical trial of KX-826 ("...
Zepp Health Corp. to Report Fourth Quarter and Full Year 2021 Financial Results on March 17, 2022
BEIJING, March 1, 2022 /PRNewswire/ -- Zepp Health Corp. ("Zepp Health" or the "Company") (NYSE: ZEPP), a cloud-based healthcare services provider with world-leading smart wearable technology, today announced that it will report its fourth quarter and full year 2021 unaudited financial results be...
PHASE II CLINICAL STUDY AT WEILL CORNELL MEDICAL CENTER INVESTIGATING PAXALISIB IN COMBINATION WITH METFORMIN AND A KETOGENIC DIET ENROLLS FIRST PATIENT
SYDNEY, Feb. 28, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that a phase II study of Kazia's investigational new drug, paxalisib, in combination with metformin and a ketogenic diet for the treatmen...
First patient treated in cohort 2 SARTATE™ neuroblastoma therapy trial
SYDNEY, Feb. 25, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that it has successfully treated its first participant in cohort ...
Terumo and Corazon Sign Collaboration and Co-Marketing Agreement
SOMERSET, N.J., Feb. 25, 2022 /PRNewswire/ -- Terumo Health Outcomes, a division of Terumo Medical Corporation, a leading global medical device manufacturer, has signed a collaboration and co-marketing agreement with Corazon, Inc., a leader in providing consulting, recruitment, interim managemen...
Daewoong Pharmaceutical Announces Successful Phase 3 Topline Results for New Antidiabetic Drug's Triple Combination Therapy
SEOUL, South Korea, Feb. 25, 2022 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) announced the topline results of the phase 3 clinical trial for a triple combination therapy of Enavogliflozin, a new antidiabetic drug with the mechanism of SGLT-2 inhibitor, with Metformin and Gemigliptin. Enav...
Week's Top Stories
Most Reposted
Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
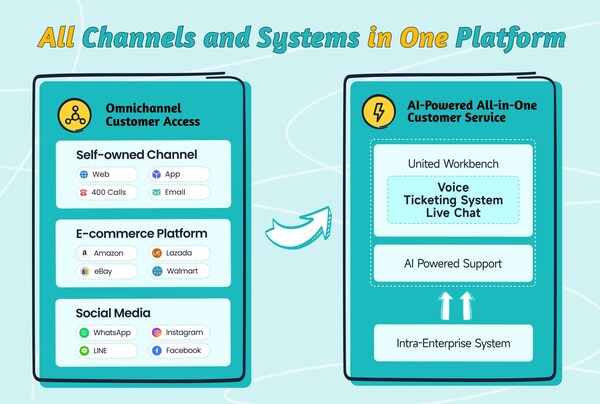
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
2024-11-11 21:00