Health
Bridge Biotherapeutics Announces China NMPA Clearance of IND for BBT-401, a Pellino-1 Inhibitor for UC
* China NMPA cleared the IND application of BBT-401 submitted on September 25, 2019 * The Phase I clinical study in China will be initiated in May 2020 SEONGNAM, South Korea, Dec. 26, 2019 /PRNewswire/ -- Bridge Biotherapeutics Inc. (KOSDAQ: 288330), a clinical stage biotech company headquart...
GenScript and Selecxine Enter Into Strategic Cooperation Agreement
SEOUL, South Korea, Dec. 26, 2019 /PRNewswire/ -- On Dec. 23, 2019, at Seoul, South Korea, the CDMO segment of the world's leading biotech company GenScript announced that it signed a strategic collaboration agreement with Selecxine Inc., a Korean biotech company dedicated to novel therapeutics f...
The 13th Dongzhi Ejiao Festival Held in Dong'e County, Shandong Province
DONG'E, China, Dec. 26, 2019 /PRNewswire/ -- On December 22, the 13th Dongzhi Ejiao Festival was held in Ejiao's birthplace, Dong'e County,Shandong Province. It was sponsored by the Herbal Paste Branch of the China Association of Chinese Medicine, the Ejiao Specialty Committee of the China Associ...
ALS Awareness Project Led by an ALS Patient
TOKYO, Dec. 24, 2019 /PRNewswire/ -- The END ALS Association (Representative: Motoi Ito, Tokyo, Japan) announced the launch of their Merry & Bright - EEG Illumination project. The objective of this project is to enable Masahiro "Hiro" Fujita,an ALS patient and founder of the association, who has ...
Viva Biotech Awarded 'Top 10 Public Companies'
BEIJING, Dec. 24, 2019 /PRNewswire/ -- The 'Future Healthcare VB 100 Summit' organized by VCBeat and VCBeat Research was held inBeijing. Viva Biotech was invited to the Summit and awarded the 'Top 10 Public Companies 2019' with honor. Dr. David Xu, the Chief Business Officer of Viva Biotech, was ...
Saluda Medical Results of U.S. Evoke Pivotal Study through 12 Months Published in The Lancet Neurology
Demonstrates Evoke® ECAP-Controlled Closed-Loop SCS Provides Statistically Superior Pain Relief Compared to Open-Loop SCS ARTARMON, Australia, Dec. 20, 2019 /PRNewswire/ -- Saluda Medical Pty Limited ("Saluda Medical") today announcedThe Lancet Neurology journal published results from the U.S. P...
China's Largest Plasmid and Virus Facility from GenScript was Put into Operation
ZHENJIANG, China, Dec. 20, 2019 /PRNewswire/ -- The CDMO segment of the world's leading biotech company GenScript announced that GenScript's plasmid and virus facility was put into operation onDecember 18, 2019. It is a milestone on the path to industrialization of the gene and cell therapy indus...
HISKY, a leader in the innovative detection technology of liver health, launches the new brand iLivTouch
BEIJING, Dec. 20, 2019 /PRNewswire/ -- Recently, Wuxi Hisky Medical Technologies Co., Ltd. (hereinafter referred to as "HISKY") has successfully launched its new brand iLivTouch based on the MigTE technology. "On the increasingly competitive market, cycles of technological innovation are key to ...
Bridge Biotherapeutics Files Investigational New Drug Application for BBT-176, an EGFR TKI for NSCLC
SEONGNAM, South Korea, Dec. 19, 2019 /PRNewswire/ -- Bridge Biotherapeutics Inc., a clinical stage biotech company headquartered in Seongnam, South Korea, announced that the company filed an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) and the Ministry...
Shenzhen signs projects worth RMB 560 billion as it promotes investment globally
SHENZHEN, China, Dec. 19, 2019 /PRNewswire/ -- Shenzhen Global Investment Promotion Conference 2019, hosted by the People's Government of Shenzhen and organized by such government entities as the Development and Reform Commission of Shenzhen Municipality, was held in the southern Chinese city on ...
Specialised Therapeutics Signs Exclusive Deal for New Haematology Drug
SINGAPORE, Dec. 18, 2019 /PRNewswire/ -- Independent pharmaceutical company Specialised Therapeutics Asia (STA) has signed an exclusive license deal with US-based Onconova Therapeutics (NASDAQ: ONTX), securing commercialisation rights to a new therapy for the treatment of Myelodysplastic Syndrome...
Dr. Jie Jack Li joins Shanghai ChemPartner as a Vice President of Discovery Chemistry
SHANGHAI, Dec. 17, 2019 /PRNewswire/ -- Shanghai ChemPartner announced the appointment ofJie Jack Li, Ph.D. as a Vice President of Discovery Chemistry at the Company headquarters inShanghai, China. Dr. Li is an established chemist with over 20 years of experience in both medicinal chemistry and ...
TransThera Biosciences Announces IND Approval from FDA for Clinical Studies of TT-00920 to Treat Chronic Heart Failure
NANJING, China, Dec. 16, 2019 /PRNewswire/ -- TransThera Biosciences Co. Ltd, a clinical-stage biotechnology company based inNanjing, China, announced today that the U.S. Food and Drug Administration (FDA) has granted the company's Investigational New Drug (IND) application for TT-00920, a novel ...
VolitionRx Limited Announces Strategic Acquisition
AUSTIN, Texas, Dec. 16, 2019 /PRNewswire/ -- VolitionRx Limited (NYSE AMERICAN: VNRX) ("Volition") today announced an agreement by its subsidiary Belgian Volition SPRL to acquire an epigenetic reagent company, Octamer GmbH ("Octamer"), for approximately$725,000 consisting of cash and shares of r...
Senhwa Biosciences Reports Positive Phase 1 Data of CX-5461 in Patients With Advanced Solid Tumors at 2019 SABCS
TAIPEI and SAN DIEGO, Dec. 12, 2019 /PRNewswire/ -- Senhwa Biosciences Inc. (TPEx: 6492), a clinical stage biopharmaceutical company focused on next generation DNA Damage Response (DDR) therapeutics for the treatment of cancer, today announced positive results from its Phase 1 trial of CX-5461. C...
United Imaging's Artificial Intelligence Subsidiary Wins in Facebook AI Research & NYU School of Medicine Global Competition
HOUSTON, Dec. 12, 2019 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, followed a strong appearance at the annual meeting of the Radiological Society ofNorth America (RSNA) with a win in a competition jointly organized by Facebook AI Researc...
iX Biopharma Reports on Outcome of Wafermine End-of-phase 2 Meeting with US FDA
SINGAPORE, Dec. 11, 2019 /PRNewswire/ -- Specialty pharmaceutical company iX Biopharma Ltd(SGX:42C) ("iX Biopharma" or, "the Company") is pleased to announce that it has successfully concluded an End-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) regarding Wafermine, a sublin...
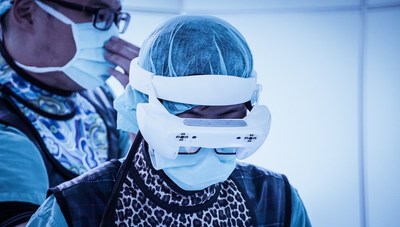
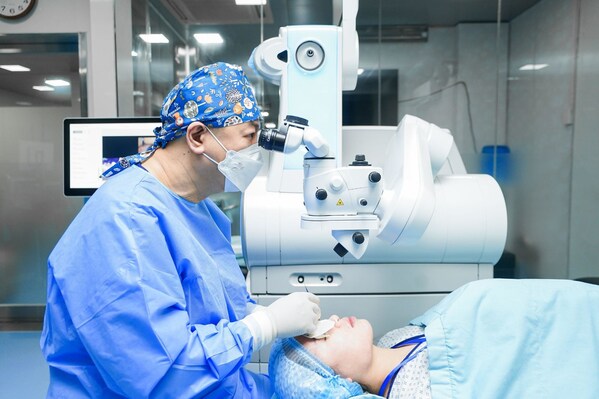
Breakthrough in Medical Electronics - A Novel Mixed & Augmented Reality Smart Glasses Surgical Navigation Solution
TAICHUNG, Dec. 10, 2019 /PRNewswire/ -- The mixed reality technology for surgery usually shown in the movies has now become a reality with the latest technological offering from theTaiwan-based company – Taiwan Main Orthopaedics Biotechnology and the branding name of medical devices is SURGLAS...
Gracell Announces Progressive Outcomes from Multiple Human Clinical Trials to Investigate FasTCAR and Dual CAR Cell Platform Technologies
SUZHOU, China and SHANGHAI, Dec. 9, 2019 /PRNewswire/ -- Gracell Biotechnologies Co., Ltd ("Gracell"), a clinical-stage immune cell therapy company, today announced the progressive clinical outcomes for leading product candidates FasTCAR-19, Dual CAR-19-22, and Dual CAR-BCMA-19 at the American...
BJ Bioscience Announces First Patient Dosed in FIH Trial of BJ-001 in Patients with Locally Advanced/Metastatic Solid Tumors
HANGZHOU, China, Dec. 9, 2019 /PRNewswire/ -- BJ Bioscience Inc. (the
'company') today announced that the first patient was successfully dosed on
December 4, 2019 in the first-in-human (FIH) trial of the company's BJ-001
program at NEXT ONCOLOGY inSan Antonio, Texas.
Week's Top Stories
Most Reposted
Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 290 media titles]
2024-11-13 09:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
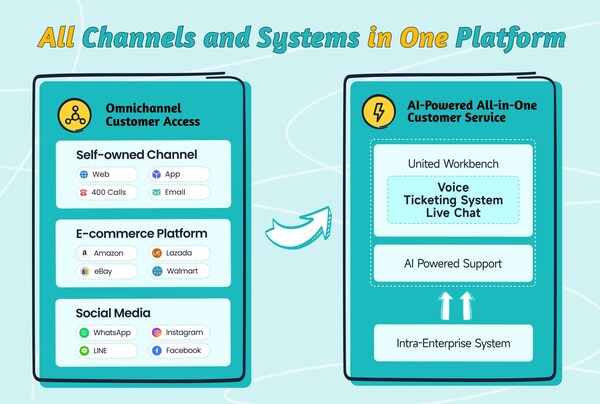
2024-11-11 21:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 289 media titles]
2024-11-12 11:00