Health
HuidaGene and Synthego Announce Licensing Agreement on Next-Generation Gene Editing Enzyme, hfCas12Max
SHANGHAI and REDWOOD CITY, Calif. , May 29, 2024 /PRNewswire/ -- HuidaGene Therapeutics ("HuidaGene"), a global clinical-stage biotechnology company focused on advancing CRISPR-based genomic medicines, and Synthego Corporation ("Synthego"), a leading provider of innovative CRISPR solutions for de...
Rege Nephro CO., Ltd. Announces Patient Enrollment for Phase II Clinical Trial of Tamibarotene for ADPKD
KYOTO, Japan, May 28, 2024 /PRNewswire/ -- Rege Nephro CO., Ltd. (
https://www.regenephro.co.jp/en
GC Cell participates 2024 BIO International Convention, Unveiling its future Blueprint 2.0, as the First Korean Business Forum Sponsor
SAN DIEGO, May 28, 2024 /PRNewswire/ -- GC Cell
AUVON Launches a New Professional TENS Unit "AUVON PT1 Pro", Offering a Drug-free Pain Relief Way at Home
Enjoy Convenient, Professional-grade, and Customizable Pain Relief with AUVON
7000
HanAll Biopharma Signs Licensing Agreement with Turn Biotechnologies to Develop Novel Treatments for Eye and Ear Diseases
* Exclusive agreement allows HanAll to utilize Epigenetic Reprogramming of Aging (ERATM) technology for ophthalmic and otic diseases. * Collaboration aims to leverage HanAll's extensive expertise in ophthalmic and otic diseases and Turn Bio's cellular reprogramming technology to create innova...
Neuren Phase 2 trial shows significant improvements in Pitt Hopkins syndrome
Highlights: * Statistically significant improvement from baseline assessed by both clinicians and caregivers in all four efficacy measures specifically designed for Pitt Hopkins syndrome (Wilcoxon signed rank test p<0.05) * Clinician and caregiver global efficacy measures showed a level of i...
Lunit to Showcase 7 Studies at ASCO 2024, including AI Innovations in HER2 Quantification, and Multimodal Predictive Models for Immunotherapy Response
- Lunit's ASCO 2024 presentations to highlight advances including HER2 ultra-low detection and AI-powered ICI response prediction models for NSCLC, demonstrating the impact of Lunit SCOPE suite on precision oncology SEOUL, South Korea, May 24, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leadin...
Mabwell Announces 9MW2821 Clinical Data and Latest Progress to be presented at 2024 ASCO Annual Meeting
SHANGHAI, May 24, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company, announced the data and latest progress of the Phase I/II clinical study of 9MW2821, a novel Nectin-4-targeting ADC for multiple advanced solid tumors, which will be reported as an oral pre...
Juncell Therapeutics Announces Clinical Data of GC203 TIL therapy in Ovarian Cancer at ASCO 2024
SHANGHAI, May 24, 2024 /PRNewswire/ -- Shanghai Juncell Therapeutics Co., Ltd. (Juncell Therapeutics), a clinical-stage biotech developing innovative Tumor-Infiltrating Lymphocyte (TIL) therapies for cancers, announced latest clinical research results on GC203 (a novel non-viral vector gene-modif...
K-Pet develops disease prevention solutions to extend the healthy life of pets
SEOUL, South Korea, May 24, 2024 /PRNewswire/ -- On the 23rd, K-PET
Tyligand Bioscience Announces First Patient Dosed in Phase 1/2 Clinical Trial of TSN1611, a Selective and Orally Bioavailable KRAS G12D Inhibitor
SHANGHAI, May 24, 2024 /PRNewswire/ -- Tyligand Bioscience, a clinical-stage biotechnology company focused on the discovery and development of innovative cancer therapies, announced that the first patient had been dosed in the Phase 1/2 trial of TSN1611 for the treatment of KRAS G12D mutant solid...
Dizal Reveals New Findings from Biomarker Analysis, Highlighting Sunvozertinib as an Effective Treatment for Non-small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations
* Anittumor efficacy of sunvozertinib was observed in patients regardless of baseline EGFR exon20ins status in plasma ctDNA. * Sunvozertinib could effectively clear EGFR exon20ins in plasma ctDNA, confirming its direct effect on EGFR pathway. * The resistance to sunvozertinib could be thro...
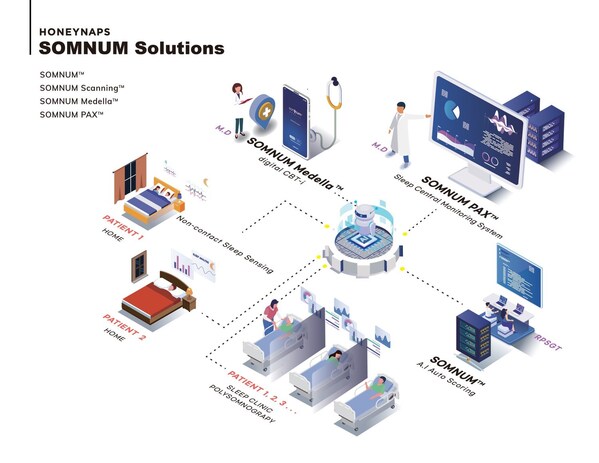
HoneyNaps secures a $11.6 million Series B investment, becoming the No. 1 ranked A.I Sleep Technology Company
HoneyNaps secures $11.6 million in series B funding, propelling its entry into the American medical market. BOSTON, May 24, 2024 /PRNewswire/ -- HoneyNaps, an industry-leading South Korean company in artificial intelligence (AI) sleep data analysis, announced on the 7th that it has closed its se...
ASCO 2024 | Ascentage Pharma Releases Latest Results from Multiple Clinical Studies of Its Lead Drug Candidates
SUZHOU, China and ROCKVILLE, Md., May 23, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that its four abstracts selected for presentations a...
Transcenta Debuts Encouraging Phase II Data from First-line Triple Combo Trial of Osemitamab (TST001) for G/GEJ Cancer at ASCO 2024
Phase II results reveal median PFS of 12.6 months in patients with CLDN18.2 high or medium expression, including in patients with PD-L1 CPS<5. PRINCETON, N.J. and SUZHOU, China, May 23, 2024 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a global clinical stage biopharm...
Cure Genetics Announced Promising Safety and Efficacy Data of CAR-NKT Product CGC729 for RCC at ASGCT 2024
SUZHOU, China, May 23, 2024 /PRNewswire/ -- On May 10, 2024, Cure Genetics announced the safety and efficacy data of their CAR-NKT product, CGC729, for patients with relapsed and refractory metastatic renal cell carcinoma (R/R mRCC) in an oral presentation at the 27th Annual Meeting of the Americ...
Computex 2024: DeCloak Intelligences Showcases DeCloakVision Multi-Modal AI Privacy-Enhanced Monitoring System Offering the Optimal Balance between Public Safety & Personal Privacy
LAS VEGAS and TAIPEI, May 23, 2024 /PRNewswire/ -- DeCloak Intelligences, a subsidiary of Etron Technology (TPEx: 5351), has won the CES Innovation Award for two consecutive years and its privacy system has also been recognized with the Excellent Manufacturer Innovation Product Award inTaiwan. De...
Complete Genomics opens U.S. supply chain to genomic sequencing customers through a new manufacturing facility at its San Jose, Calif. headquarters
SAN JOSE, Calif., May 23, 2024 /PRNewswire/ -- Complete Genomics, a pioneering genomic sequencing company, recently welcomed vendors, suppliers, customers and employees to aMay 17 grand opening celebration of its 10,115 square foot manufacturing facility at its headquarters inSan Jose, California...
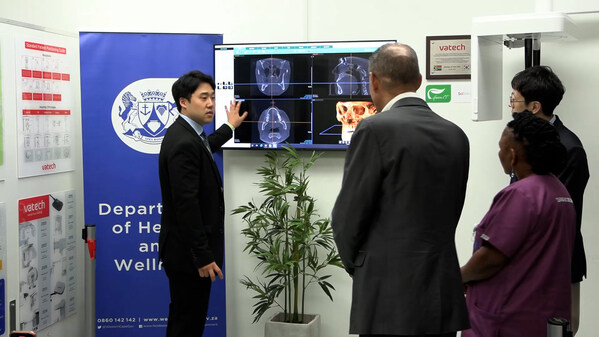
Vatech Partners with Tygerberg Public Hospital to Improve Dental Care in South Africa
SEOUL, South Korea, May 23, 2024 /PRNewswire/ -- Vatech, a global leader in the dental imaging market, is launching a project to improve dental healthcare in South Africa. Vatech announced today that it has partnered with Tygerberg Hospital inCape Town, South Africa to donate medical equipment for...
Laekna Announces FDA Approval for the Phase III Clinical Trial Protocol of LAE002 (Afuresertib) PLUS LAE001 for the Treatment of Prostate Cancer
SHANGHAI and WARREN, N.J., May 23, 2024 /PRNewswire/ -- Laekna, Inc. (2105.HK), a science-driven, clinical-stage biotechnology company, announced that the company has received approval from the U.S. Food and Drugs Administration for the protocol of the phase III clinical trial of LAE002 (afureser...
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 293 media titles]
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
2024-11-11 21:00Oxygen, HubSpot's leading solutions partner in Hong Kong & Greater China Expands to UAE
[Picked up by 285 media titles]
2024-11-15 15:25