Health
DP Technology Announces Nomination of Development Candidate, a Potential Best-in-Class Kv1.3 Inhibitor for Immunological Diseases Including IBD and AD
* The nomination of novel Kv1.3 inhibitor DPT0218 for the treatment of various immunological diseases including IBD and AD. • DPT0218 is a gut-restricted, highly potent and selective Kv1.3 inhibitor with excellent in vivo efficacy and safety profile in preclinical studies. * This project fur...
Vesicure Therapeutics made notable advancements using exosomes to target KRAS mutation
SUZHOU, China, Jan. 29, 2024 /PRNewswire/ -- SiRNA technology offers distinct advantages over conventional approaches, with the potential to target virtually any gene. This technology has emerged as a great strategy for treating a range of diseases, including cancer, viral infections, and genetic...
Oricell Announces FDA Clearance of IND Application for OriCAR-017, a novel GPRC5D Targeted CAR-T Cell Therapy Utilizing the Company's Proprietary Platform, for the Treatment of Relapsed/Refractory Multiple Myeloma.
SHANGHAI and ROSELAND, N.J., Jan. 29, 2024 /PRNewswire/ -- Oricell Therapeutics (Oricell), a clinical-stage biotechnology company, today announced that the U.S. Food and Drug Administration (FDA) has cleared the company's Investigational New Drug (IND) application for OriCAR-017 for patients with...
Peijia Medical Announces Successful Completion of Patient Enrollment for Pivotal TaurusTrio™ Transcatheter Aortic Regurgitation Clinical Trial
SUZHOU, China, Jan. 29, 2024 /PRNewswire/ -- Peijia Medical Limited (Peijia, (9996.HK)), a leading Chinese domestic player in the high-growth transcatheter valve therapeutic and neurointerventional procedural medical device markets, today announced the completion of patient enrollment and implant...
Ractigen Therapeutics Enters Strategic Partnership with University Medical Center Utrecht to Drive saRNA Innovation in Neurodevelopmental Disorders
SUZHOU, China, Jan. 29, 2024 /PRNewswire/ -- Ractigen Therapeutics, a clinical-stage pharmaceutical company leading the charge in small activating RNA (saRNA) therapeutics, announces a formal collaboration with University Medical Center Utrecht, affiliated withUtrecht University. This strategic ...
Crystal Bio Welcomes Dr. Shiaw-Lin (Billy) Wu as Co-Founder and Chief Scientific Officer
CRANBURY, N.J., Jan. 29, 2024 /PRNewswire/ -- Crystal Bio, an innovative spinout division of Crystal Pharmatech, is excited to announce Dr.Shiaw-Lin (Billy) Wu as its new co-founder and Chief Scientific Officer (CSO). Dr. Wu brings with him more than three decades of experience in the field of...
Asieris Releases Phase III Clinical Study and Real-World Research Data for APL-1706, a Bladder Cancer Diagnosis and Management Drug, at the 2024 ASCO-GU
SHANGHAI, Jan. 29, 2024 /PRNewswire/ -- Asieris Pharmaceuticals (Stock Code: 688176.SH), a global biopharmaceutical company specializing in discovering, developing, and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced the release of ...
Asieris Presents for the First Time Interim Analysis Data of Oral APL-1202 in Combination with PD-1 Inhibitor Tislelizumab for Neoadjuvant Treatment of Muscle-Invasive Bladder Cancer at 2024 ASCO-GU
SHANGHAI, Jan. 29, 2024 /PRNewswire/ -- Asieris Pharmaceuticals (Stock Code: 688176.SH), a global biopharmaceutical company specializing in discovering, developing, and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced the first-time ...
COSMAX Established OTC Lab…'Targeting the $2.6 Billion U.S. sun care market'
- Actively responding to its global clients targeting the U.S. sun care market - Strengthening the K-Sunscreen formulations lineup including cushions and sticks - Enhancing its expertise in sun care products through OTC Lab SEONGNAM, South Korea, Jan. 26, 2024 /PRNewswire/ -- COSMAX, a global cos...
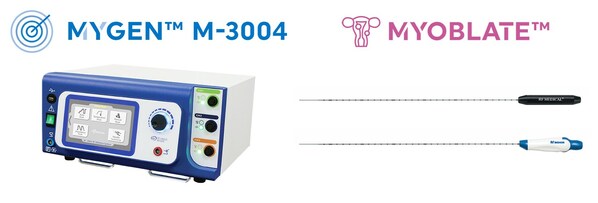
FDA clears RF Medical's MYGEN™ M-3004 and MYOBLATE™ Radiofrequency Ablation System
SEOUL, South Korea, Dec. 12, 2022 /PRNewswire/ -- RF Medical Co., Ltd. is a
premier Korean-based medical company serving the healthcare industry for almost
two decades. Recently, FDA cleared RF Medical's patented MYGEN™ M-3004
generator and MYOBLATE™ radiofrequency ablation system.
Endomondo.com is Back Online - As a Professional Fitness Guidance Website
SAN JOSE, Calif, Jan. 25, 2024 /PRNewswire/ -- Endomondo.com, a fitness and
wellness industry pioneer, is excited to announcea significant transformation
as it evolves into a leading professional fitness guidance website
Glenmark, Jiangsu Alphamab Biopharmaceuticals and 3D Medicines announce the signing of a License Agreement for KN035 (Envafolimab) for Multiple Geographies around the World
* Glenmark will be responsible for further developing, registering, and commercializing Envafolimab inIndia, Asia Pacific, Middle East and Africa, Russia, CIS, and Latin America. * Jiangsu Alphamab and 3D Medicines will receive a low double digit Million US Dollar amount up to launch, additio...
Asieris will Present For the First Time Interim Analysis Data of Oral APL-1202 in Combination with PD-1 Inhibitor Tislelizumab for Neoadjuvant Treatment of Muscle-Invasive Bladder Cancer at 2024 ASCO-GU
SHANGHAI, Jan. 25, 2024 /PRNewswire/ -- Asieris Pharmaceuticals (Stock Code: 688176.SH), a global biopharmaceutical company specializing in discovering, developing, and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced that the interi...
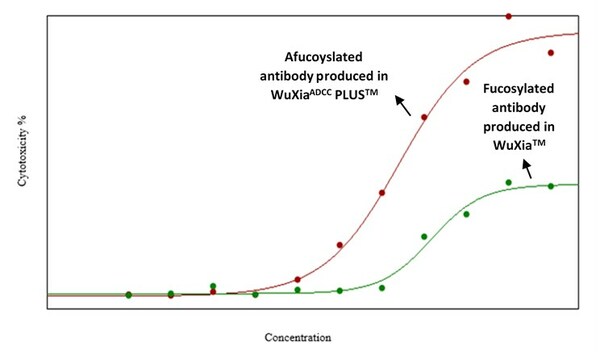
WuXi Biologics Launches WuXia ADCC PLUS™ for the Development and Manufacturing of Afucosylated Antibodies that Elicit Enhanced ADCC Effect
- WuXiaADCC PLUSTM is designed to meet the market need for improved antibody therapeutic efficacy by producing afucosylated antibodies with the ability to increase antibody-dependent cell-mediated cytotoxicity (ADCC), providing diverse bioprocessing solutions for global clients - WuXiaADCC PLU...
Wellysis Goes Global with ECG Monitor 'S-Patch', Enters US and Indian Markets
SEOUL, South Korea, Jan. 24, 2024 /PRNewswire/ -- Digital healthcare innovator Wellysis is making waves in the global market with its revolutionary electrocardiogram (ECG) monitoring solution, 'S-Patch,' securing strategic partnerships and distribution deals to propel its entry into the lucrative...
WestGene Spearheads Oncology Breakthroughs at the 3rd mRNA-Based Therapeutics Summit
CHENGDU, China, Jan. 24, 2024 /PRNewswire/ -- Kicking off the New Year with remarkable achievements,Chengdu-based WestGene made a significant impact at the 3rd mRNA-Based Therapeutics Summit inBerlin, Germany. Renowned for her pivotal role in mRNA research, Dr.Xiangrong Song, co-founder and CEO o...
Positive Xanamem® human PET study published in the Journal of Alzheimer's Disease demonstrating robust CNS[1] target enzyme occupancy
The study confirms that Xanamem is a brain-penetrant inhibitor of the tissue cortisol synthesis enzyme, 11β-HSD1, with high levels of target occupancy at doses as low as 5 mg SYDNEY, Jan. 24, 2024 /PRNewswire/ -- Actinogen Medical Limited (ASX: ACW) announces that its human Positron Emission Tom...
Genome & Company Announces Positive Topline Results from Phase 2 Clinical Trial of Combination of GEN-001 and Bavencio® for the Treatment of Gastric Cancer Poster presented at ASCO GI
* Results showed partial response being confirmed in 7 out of 42 patients * Partial response was observed in 3 out of 8 patients who were previously treated by immunotherapy SEOUL, South Korea, Jan. 24, 2024 /PRNewswire/ -- Genome & Company (KOSDAQ: 314130), a clinical stage biotech leading in...
PRISM BioLab raises 1.5 billion yen in Series C fundraising to advance technologies and programs targeting protein-protein interactions
Eli Lilly and Company, Santec Holdings Corporation participated in the round TOKYO, Jan. 24, 2024 /PRNewswire/ -- TOKYO, Jan. 24, 2024 /PRNewswire/ -- PRISM BioLab, Co. Ltd. ("PRISM"), a leading discovery and development biotechnology company designing small molecule inhibitors of protein-protei...
Harbour BioMed Announces IND Clearance for HBM9027 in the U.S.
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Jan. 23, 2024 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142) announced that the Company has been granted the clearance of Investigational New Drug (IND) from the Food and Drug Administration (FDA) ofthe United States to init...
Week's Top Stories
Most Reposted
Mastercard goes OTP-free in APAC for faster, safer online transactions
[Picked up by 326 media titles]
2024-11-06 09:00Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 308 media titles]
2024-11-12 09:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 288 media titles]
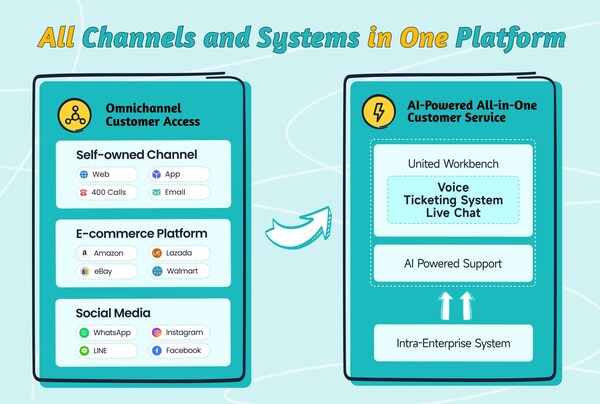
2024-11-11 21:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 284 media titles]
2024-11-12 11:00