Health
Meihua International Medical Technologies Co., Ltd. Announces Initial $6 Million Tranche of Potential $50.5 Million Maximum Offering
YANGZHOU, China, Dec. 28, 2023 /PRNewswire/ -- Meihua International Medical Technologies Co., Ltd. ("MHUA" or the "Company") (NASDAQ: MHUA), a reputable manufacturer and provider of Class I, II and III disposable medical devices with operating subsidiaries inChina, announced today that it has ent...
Heranova Lifesciences Debuts with $13.5 Million Seed and Seed+ Round Funding to Offer Integrated Clinical Solutions to Women's Health
BOSTON and HONG KONG, Dec. 28, 2023 /PRNewswire/ -- Heranova Lifesciences Holding Ltd. ("Heranova") today announced the successful completion of aUSD 13.5 million seed and seed+ financing to develop and offer integrated Women's Health solutions worldwide. This funding will be used to launch a ...
Caliway to Present CBL-514 Phase 2 Study Results for Subcutaneous Fat Reduction at IMCAS 2024
CBL-514 Phase 2 Study Results for local fat reduction demonstrated 85.7% and 76.2% of participants lost at least 150mL and 200mL of abdominal subcutaneous fat in the treated area after receiving CBL-514 treatment(s). NEW TAIPEI CITY, Dec. 26, 2023 /PRNewswire/ -- Caliway Biopharmaceuticals (Cal...
PharmaBlock Opens HPAPI GMP Facility at Zhejiang Manufacturing Site
NANJING, China, Dec. 26, 2023 /PRNewswire/ -- PharmaBlock Sciences (Nanjing), Inc. (Stock code: 300725.SZSE), a global, fully integrated CRDMO company, focusing on innovative chemistry and low-carbon manufacturing, has announced opening of a new high potency API (HPAPI) GMP facility (OEB-5 and ab...
Jemincare Announces 6 Approvals of Clinical Trials for its Innovative Drugs
SHANGHAI, Dec. 26, 2023 /PRNewswire/ -- Jemincare, a leading pharmaceutical company fromChina, announced that its wholly owned subsidiary company, Shanghai Jemincare Pharmaceutical Co., Ltd., recently received 6 approvals of clinical trials for its innovative drugs in the field of cancer, kidney ...
PharmaBlock Obtained ISO 50001 Certification, Advancing Sustainable Development
NANJING, China, Dec. 25, 2023 /PRNewswire/ -- PharmaBlock Sciences (Nanjing), Inc. (Stock code: 300725. SZSE), a global, fully integrated CRDMO company, focusing on innovative chemistry and low-carbon manufacturing, has announced the successful attainment of Energy Management System Certification...
Harbin City Pushes for Breakthroughs in Full Revitalization of Northeast China
HARBIN, China, Dec. 25, 2023 /PRNewswire/ -- Harbin, capital of Heilongjiang Province, has strived to build itself into a city of innovation, by sticking to reform and innovation and stimulating endogenous strength to push for breakthroughs in the full revitalization of northeastChina. The Harbi...
FDA Keynote Speaker in the Spotlight, CAR-T Industry Leaders Gather for Discussion-Join the 2024 GenScript Biotech Global Forum for a New Journey in Cell and Gene Therapy
NANJING, China, Dec. 24, 2023 /PRNewswire/ -- The innovative development of biopharmaceuticals has ushered in a new era for disease treatment, bringing unprecedented hope to countless patients. However, the transition from basic research to clinical commercialization presents formidable challenge...
APL-2301, a compound developed by Asieris for the treatment of Acinetobacter baumannii infection, was approved for Phase I clinical trials in Australia
SHANGHAI, Dec. 24, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (Stock Code: 688176.SH), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced that its product, APL-23...
GenSci Launches Global Innovation Hub in Shanghai
The Establishment of the Facility Demonstrates the Company's Commitment to Delivering Differentiated Solutions to Gynecology and Pediatrics Challenges SHANGHAI, Dec. 22, 2023 /PRNewswire/ -- Changchun GeneScience Pharmaceutical ("GenSci"), a subsidiary of Changchun High-Tech Industries (Group), h...
Yoshitsu Co., Ltd Reports First Six Months of Fiscal Year 2024 Financial Results
TOKYO, Dec. 22, 2023 /PRNewswire/ -- Yoshitsu Co., Ltd ("Yoshitsu" or the "Company") (Nasdaq: TKLF), a retailer and wholesaler of Japanese beauty and health products, sundry products, luxury products, electronic products, as well as other products inHong Kong, mainland China, Japan, North America...
TransThera Announces the Global Multicenter Phase 3 Clinical Trial Completed First Patient Dosing in the US Evaluating Tinengotinib in FGFRi Relapsed/Refractory Patients with Cholangiocarcinoma
NANJING, China and GAITHURSBURG, Md., Dec. 21, 2023 /PRNewswire/ -- TransThera, a clinical-stage biopharmaceutical company dedicated to innovating differentiated drugs globally, today announces the first patient has been dosed in the US for the Phase 3 trial FIRST-308 of tinengotinib (TT-00420), ...
BioCity announces the first patient dosed with its anti-TIM-3 mAb BC3402 in Combination with IMFINZI for the Treatment of Advanced Hepatocellular Carcinoma in a Phase Ib/II Trial
WUXI, China, Dec. 20, 2023 /PRNewswire/ -- BioCity Biopharma today announced dosing of the first patient in a Phase Ib/II clinical trial of its anti-TIM-3 monoclonal antibody (mAb) BC3402 in combination with IMFINZI (durvalumab) for the treatment of advanced hepatocellular carcinoma (HCC) inCh...
YS Biopharma Responds to Unauthorized Press Release Regarding Extraordinary General Meeting
GAITHERSBURG, Md., Dec. 20, 2023 /PRNewswire/ -- YS Biopharma Co., Ltd. (NASDAQ: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious disea...
/DISREGARD RELEASE: YS Biopharma/
We are advised by YS Biopharma Co., Ltd. (NASDAQ:YS) that journalists and other readers should disregard the news release, "YS Biopharma Co., Ltd. to Hold Extraordinary General Meeting onDecember 28, 2023", issued on December 20, 2023 by YS Biopharma over PR Newswire, as the release contained err...
CellOrigin announced treatment of the first patient with CAR-M in China and reported the second generation of CAR-M for solid tumors
HANGZHOU, China, Dec. 20, 2023 /PRNewswire/ -- Recently, a CAR-macrophage therapy product (CAR-M) SY001 from CellOrigin Biotechnology (Hangzhou) Co., Ltd. was dosed to the first patient in a hospital inChina. "A single center, single-arm, dose-escalation, exploratory clinical trial to examine t...
Genevoyager Unveils Gene Therapy CDMO Facility in Milestone Move
PHILADELPHIA, Dec. 20, 2023 /PRNewswire/ -- Genevoyager (Wuhan) Co., Ltd. (Genevoyager), a leading provider of one-stop CRO/CDMO services for gene therapy products, proudly announces the official opening of its Contract Development and Manufacturing Organization (CDMO) facility. This significant ...
HanAll Biopharma, Daewoong Pharmaceutical and NurrOn Pharmaceuticals to Showcase Ongoing Parkinson's Disease Program at the 7th Annual Sachs Associates Neuroscience Innovation Forum
ROCKVILLE, Md., Dec. 20, 2023 /PRNewswire/ -- HanAll Biopharma (KRX: 009420.KS), Daewoong Pharmaceutical (KRX: 069620.KS), and NurrOn Pharmaceuticals announced their participation in the 7th Annual Sachs Associates Neuroscience Innovation Forum. The event is scheduled to take place onJanuary 7th...
InxMed Enters License Agreement with Escugen to Develop Next-Generation ADCs
NANJING, China, Dec. 19, 2023 /PRNewswire/ -- InxMed, a clinical-stage biotechnology company dedicates on developing innovative therapies against drug resistance, and Escugen, a clinical-stage antibody–drug conjugate (ADC) company, today announced that InxMed licensed EZWi-Fit® linker-payload pl...
Standigm named as a Tech Innovator in Generative AI in Drug Discovery use case
SEOUL, South Korea, Dec. 19, 2023 /PRNewswire/ -- Standigm
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
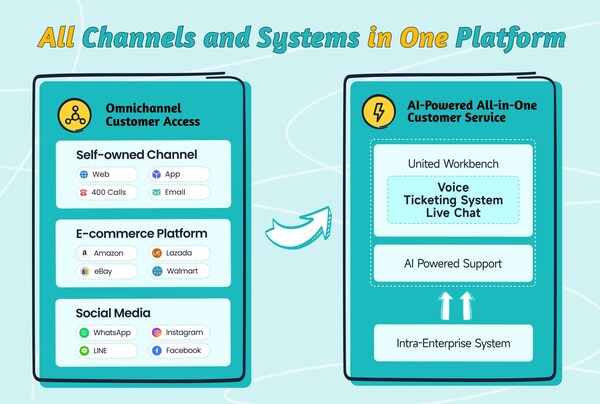
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
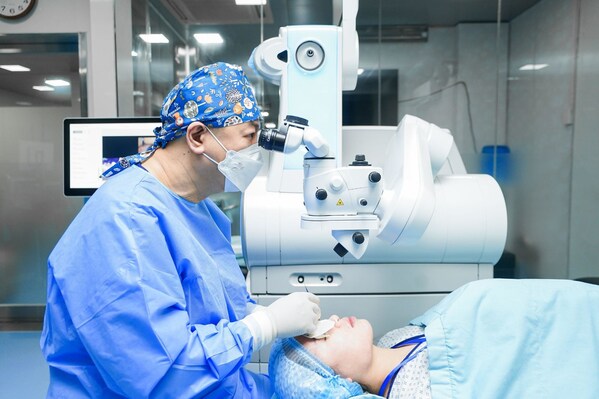
2024-11-11 21:00DND International Eye Hospital: Pioneering SMILE Pro Surgery & Leading the Trend of Refractive Surgery Tourism in Vietnam
[Picked up by 279 media titles]
2024-11-08 20:11