Health
Dizal Announces Sunvozertinib Meets Primary Endpoint in its First Pivotal Study in Platinum-Pretreated NSCLC Patients with EGFR Exon20ins Mutations at 2022 ESMO
* The confirmed objective response (cORR), as assessed by blinded independent central review (BICR), was 59.8% in advanced NSCLC with EGFR exon20ins mutations after at least one line of platinum-based chemotherapies * The response rate for patients with baseline brain metastasis was 48.4% * ...
Dizal Announces Positive Phase I Clinical Trial Results for DZD1516 in Treating HER2 Breast Cancer at 2022 ESMO
PARIS, Sept. 5, 2022 /PRNewswire/ -- Dizal reported the promising safety and pharmacokinetic data from the global Phase I study ofDZD1516 in patients with HER2 positive metastatic breast cancer (HER2+ MBC) who relapsed from multiple prior treatments at the 2022 European Society for Medical Oncolo...
Recruitment opens for Phase II trial in prostate cancer with Cu-64 SAR-Bombesin in the US
SYDNEY, Sept. 5, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the US-based diagn...
Lunit to Showcase 5 Abstracts at ESMO 2022
* Lunit to present five posters at ESMO 2022 featuring the company's AI-biomarker platform * Studies corroborate the capability of Lunit SCOPE suite to address an expanding set of clinical and research questions based on world-leading digital pathology AI SEOUL, South Korea, Sept. 5, 2022 /PR...
Vernalis Research, a fully owned subsidiary of HitGen Inc., and Unison Medicines Inc. announce a research collaboration in the field of anti-infectives
CHENGDU, China, Sept. 5, 2022 /PRNewswire/ -- Vernalis Research ("Vernalis"), a fully owned subsidiary of HitGen Inc., and Unison Medicines Inc. ("Unison") are pleased to announce a research collaboration on an undisclosed bacterial target. Under the terms of the agreement, Vernalis will use it...
Simcere Pharmaceutical Announces Financial Results for 2022 H1: 27% Year-Over-Year Revenue Growth, with Innovative Drugs Accounting for 65.4% of Total Revenue
NANJING, China, Sept. 5, 2022 /PRNewswire/ -- Simcere Pharmaceutical Group Limited (2096.HK) announced its financial results for the first half of 2022. As ofJune 30, Simcere recorded operating revenue of RMB 2.7 billion for the first half of the year, with a year-over-year growth of 27.3%. The e...
Tamas Oravecz Ph.D. named SVP, CSO of the U.S. of Simcere Pharmaceutical Group
NANJING, China, Sept. 5, 2022 /PRNewswire/ -- Simcere Pharmaceutical Group announces today the appointment ofTamas Oravecz, Ph.D. as Senior Vice President, Chief Scientific Officer of the Simcere U.S. Tamas will be responsible for providing strategic leadership to our drug discovery efforts in A...
New Building Stands as Symbol of Dignity and Hope For Persons Affected by Leprosy in Ethiopia
TOKYO, Sept. 2, 2022 /PRNewswire/ -- Sasakawa Health Foundation has funded the
construction of a building inAddis Ababa for the Ethiopian National Association
of Persons Affected by Leprosy (ENAPAL).
Neukio Biotherapeutics completed Series A-1 financing, to accelerate discovery and development of next generation cell therapy products
SHANGHAI, Sept. 2, 2022 /PRNewswire/ -- Neukio Biotherapeutics, a company committed to developing novel cell therapy products, announces it has closed $50 million in a Series A-1 funding round. The investment round was led by CD Capital, with the participation of Alwin Capital and Surplus Capital...
Waterdrop Inc. to Report Second Quarter 2022 Financial Results on September 9, 2022
BEIJING, Sept. 2, 2022 /PRNewswire/ -- Waterdrop Inc. (NYSE: WDH) ("Waterdrop" or the "Company"), a leading technology platform dedicated to insurance and healthcare service with a positive social impact, today announced that it will report its unaudited financial results for the second quarter e...
Jacobio Completes First Patient Dosage of CD73 mAb JAB-BX102 in China
BEIJING, Sept. 2, 2022 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced it has completed first patient dosage of it's in-house R&D drug candidate CD73 monoclonal antibody JAB-BX102 in a Phase I/IIa clinical trial for advanced solid tumour patients. This is a phase I/IIa multi-center, open-la...
Jacobio Completes First Dose of IIa Clinical Trial of JAB-21822 on KRAS G12C and STK11 Co-mutation in China
BEIJING, Sept. 1, 2022 /PRNewswire/ -- JacobioPharma (1167.HK) announced that the company has dosed the first non-small cell lung cancer (NSCLC) patient with KRAS G12C and STK11 co-mutation in the Phase IIa trial of KRAS G12C inhibitor JAB-21822 inChina. JAB-21822 is a KRAS G12C inhibitor inde...
Bridge Biotherapeutics Presented Non-clinical Study Results for 2 IPF Candidates at the IPF Summit 2022
* Non-clinical studies explored the potent anti-fibrotic and anti-inflammatory efficacy of BBT-301 and BBT-209 for IPF treatment * Company enhances its strategic focus on fibrotic diseases inclusive of IPF with 1 clinical asset and 2 non-clinical assets BOSTON and SEONGNAM, South Korea ,Sept. ...
Pxierra is launching world's first non-contact heart rate & breathe rate measurement AI baby monitor
NEW YORK, Sept. 1, 2022 /PRNewswire/ -- Pxierra is excited to announce the launch of its innovative AI Baby monitor. This new device is designed to provide non-contact heart rate and breathing rate measurement, making it easier and more convenient for parents to keep track of their newborns' vita...
Zhongchao Inc. Announces its New Strategy Extension Focusing on the Oncology and Other Major Disease Management
SHANGHAI, Sept 1, 2022 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), an internet technology company offering healthcare professionals the online healthcare information, professional training and educational services platform and patient management service, today a...
The ECO-Friendly Anti-nausea and Vomiting wristband: Emeterm PLUS
VANCOUVER, BC, Aug. 31, 2022 /PRNewswire/ -- WAT Medical is thrilled to introduce its latest innovative technology that would help the millions of people who suffer from nausea and vomiting, the EmeTerm Plus, while also developing an eco-friendly roadmap that would benefit future innovations. The...
AI Startup AESOP Raises $3M to Tackle Medical and Billing Errors
SAN FRANCISCO, Aug. 31, 2022 /PRNewswire/ -- Digital health startup AESOP
Technology
WuXi Biologics Announces GMP Release of Its First North American Biomanufacturing Facility in Cranbury, New Jersey
CRANBURY, N.J., Aug. 31, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global leading Contract Research, Development and Manufacturing Organization (CRDMO), today announced the release of its GMP phase I drug substance clinical manufacturing facility, MFG18, inCranbury, New Jersey...
Licensing and Agency Agreement Signed between Beijing Minhai (Biokangtai) and Phil. Pharmawealth, Inc.
Marks the First Entry of Biokangtai's 13-valent Pneumococcal Vaccine into Southeast Asian Market SHENZHEN, China, Aug. 31, 2022 /PRNewswire/ -- On August 30, Beijing Minhai Biotechnology Co., Ltd. held a signing ceremony with Phil. Pharmawealth, Inc. for their cooperation in jointly promoting th...
Nreal Air AR Glasses Receive TÜV Rheinland Eye Comfort (AR) Certification
SHANGHAI , Aug. 30, 2022 /PRNewswire/ -- On August 22, 2022, TÜV Rheinland Greater China ("TÜV Rheinland") awarded the Eye Comfort (AR) Certification for Nreal's AR glasses, Nreal Air, ahead of the device's official launch inChina. The bestowal of TÜV Rheinland's highest-level display certificati...
Week's Top Stories
Most Reposted
Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
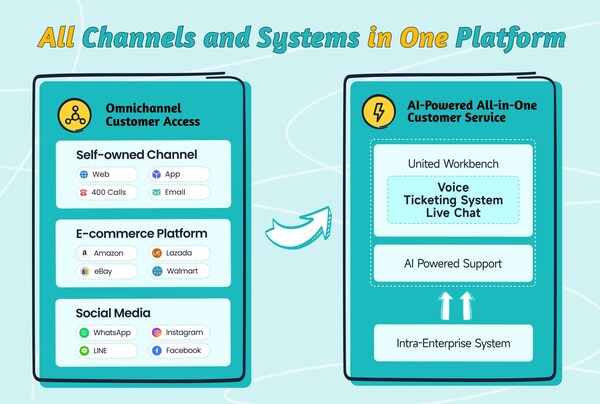
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
2024-11-11 21:00