Health
Jacobio Pharma Receives IND Approval for P53 Y220C Activator JAB-30300 in the U.S.
BEIJING, SHANGHAI and BOSTON, March 1, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced it received IND (Investigational New Drug) approval of its self-developed drug JAB-30300 (P53 Y220C activator) from the FDA of ...
SN BioScience received US FDA Orphan Drug Designation for its Nano Anti-Cancer Drug 'SNB-101' on Pancreatic Cancer
SEOUL, South Korea, Feb. 29, 2024 /PRNewswire/ -- SN Bioscience Co. Ltd. (CEO Park Young-hwan) announced onFeb 27 that the US FDA had granted an orphan drug designation for pancreatic cancer for SNB-101 (API: SN-38), a new polymer nanoparticle drug under development, based on the pre-clinical dat...
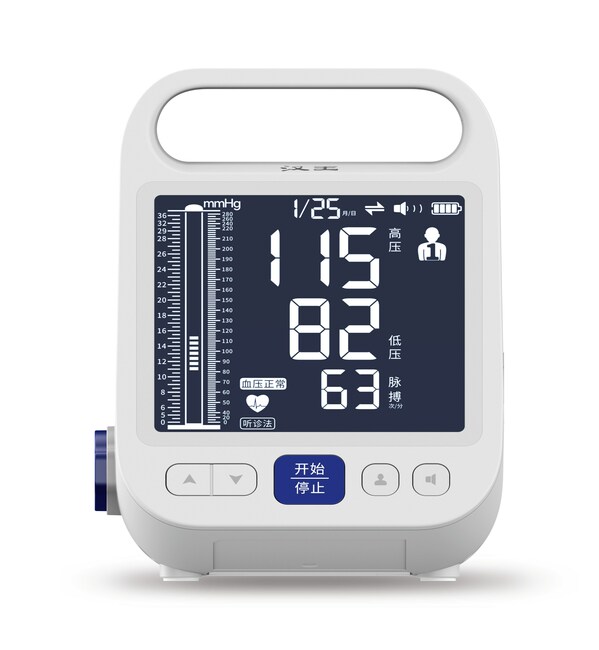
Hanvon Technology Launches Groundbreaking Fourth-Generation BP Meter Based on Korotkoff Sound Method
A World Health Organization report highlights that a third of the global adult population suffers from hypertension, with nearly half undiagnosed due to inadequate blood pressure meters, posing significant health risks. BEIJING, Feb. 28, 2024 /PRNewswire/ -- Hanvon Technology Co., Ltd., based in ...
Chamlion and 3DRPD Forge Collaboration for Dental 3D Production Center
CHICAGO, Feb. 28, 2024 /PRNewswire/ -- LAB DAY® Chicago 2024 witnessed a new collaboration announcement between Chamlion, a global leader in integrated dental 3D printing solutions, and 3DRPD, a renowned pioneer of 3D printed RPDs. The highlight is to make the announcement of their collaboration ...
Lunit Presents Seven Study Results at ECR 2024: Showcasing AI's Robust Performance in Diverse Clinical Settings
- Lunit to unveil four oral presentations and three poster presentations at ECR 2024, highlighting Lunit INSIGHT's expansive capability, ranging from adaptability in different use cases to the potential to replace a human reader in mammography double-reading settings SEOUL, South Korea, Feb. 28...
PharmAust announces positive Phase 1 MEND Study Top-Line Results in MND / ALS
Highlights: * Monepantel displays a superior safety, tolerability to the leading FDA approved drug Relyvrio® * Preliminary efficacy data shows a 58% reduction in the rate of disease progression for Cohort 2 (High Dose) using the FDA primary efficacy endpoint, ALSFRS-R * Confirmation that m...
Baird Medical Expands Presence in the United States
FRISCO, Texas, Feb. 28, 2024 /PRNewswire/ -- Baird Medical Devices, Inc. ("Baird Medical" or the "Company"), a leading microwave ablation ("MWA") medical device developer and provider inChina and the United States, today announced that it is accelerating its expansion inthe United States through ...
The promising results of a phase II clinical study for Akeso's Cadonilimab (PD-1/CTLA-4) combined standard treatment for first-line treatment of R/M cervical cancer has been published in Clinical Cancer Research
HONG KONG, Feb. 28, 2024 /PRNewswire/ -- Akeso (9926.HK) announced that the results of a phase II clinical trial for PD-1/CTLA-4 bispecific antibody(cadonilimab)combined with standard treatment (chemotherapy +/- bevacizumab) as first-line treatment for recurrent/metastatic cervical cancer had be...
China Jo-Jo Drugstores, Inc. Announces 1-for-20 Reverse Stock Split
HANGZHOU, China, Feb. 27, 2024 /PRNewswire/ -- China Jo-Jo Drugstores, Inc. (the "Company") (Nasdaq: CJJD), a Cayman Islands exempted company, reported that it expects to implement a 1-for-20 reverse stock split on its ordinary shares ("Ordinary Shares") effective Friday, March 1, 2024, with trad...
FDA Grants Fast Track Designation to 9MW2821
SHANGHAI, Feb. 27, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announces that its self-developed novel ADC drug targeting Nectin-4 (R&D Code: 9MW2821) has been granted Fast Track Designation (FTD) by the U.S. Food and Drug Administ...
111 Announces Receipt of Withdrawal of Going Private Proposal
SHANGHAI, Feb. 27, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced that it has received a notice dated Feburary 27, 20...
Origin Agritech Announces Breakthrough in Corn Hybrid Development with Wild Corn Gene Integration
BEIJING, Feb. 27, 2024 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), a leading Chinese agricultural technology company, today announced a breakthrough development in its commercial corn hybrid offerings. Origin Agritech has successfully integrated a gene from wi...
Zhongchao Inc. Announces 1-for-10 Share Consolidation
SHANGHAI, Feb. 27, 2024 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), a platform-based internet technology company offering services for patients with cancer and other major diseases, today announced that an extraordinary general meeting of shareholders held onTues...
Nona Biosciences Enters into Collaboration Agreement with Boostimmune in Antibody-Drug Conjugate Development
CAMBRIDGE, Mass., Feb. 26, 2024 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited committed to cutting-edge technology innovations and providing a total solution from "Idea to IND" ("I to ITM"), announced today that it has entered into a collaboration agreement w...
BioCity Announces Enrollment Completion of the IgA Nephropathy (IgAN) Cohort in the Randomized, Placebo-controlled Phase II Clinical Trial of the ETA Receptor Antagonist SC0062
WUXI, China, Feb. 25, 2024 /PRNewswire/ -- BioCity Biopharma is pleased to announce the completion of enrollment of all 120 participants in the IgA nephropathy (IgAN) cohort in a randomized, double-blind, placebo-controlled Phase 2 clinical study of the novel, oral endothelin A (ETA)-receptor sel...
GC Cell and BioCentriq® Execute Process Transfer Agreement in Anticipation of the U.S. entry of Immuncell-LC Inj.
* Immuncell-LC Inj. is an autologous Cytokine Induced Killer (CIK) cell therapy approved for Commercial use inSouth Korea as an adjuvant cell therapy for the treatment of Hepatocellular Carcinoma (HCC) after curative resection and has receivedFDA Orphan Drug Designations (ODDs) for liver, brain...
Aucta Pharmaceuticals, Inc. ("Aucta Pharmaceuticals") launches MOTPOLY XR™ (lacosamide) extended-release capsules C-V, the first, and only once-daily formulation of lacosamide
PISCATAWAY, N.J., Feb. 26, 2024 /PRNewswire/ -- Aucta Pharmaceuticals, Inc., a private specialty pharmaceutical company focused on niche generic and branded specialty products, today announced the commercial launch of MOTPOLY XR (lacosamide) extended-release capsules C-V (100, 150 and 200 mg). M...
TransThera initiates IND-Enabling studies for TT-02332, a novel, highly potent and CNS-penetrating NLRP3 inhibitor
NANJING, China and GAITHURSBURG, Md., Feb. 26, 2024 /PRNewswire/ -- TransThera, a clinical-stage biopharmaceutical company dedicated to innovating differentiated drugs globally, today announced the initiation of IND-enabling studies for TT-02332, a potent, selective and highly CNS-penetrating NLR...
J INTS BIO, Phase 1/2 study of 'JIN-A02', a Novel Oral 4th Generation EGFR TKI, accepted for presentation at the upcoming American Association for Cancer Research 2024 meeting in USA (AACR 2024)
SEOUL, South Korea, Feb. 26, 2024 /PRNewswire/ -- J INTS BIO announced on the 26th of month that the Phase 1/2 clinical study of its Novel Oral 4th Generation EGFR TKI "JIN-A02" for the treatment of NSCLC has been accepted for poster presentation at the upcoming American Association for Cancer Re...
Groundbreaking! Phase II Data of LP-003 Unveiled by Longbio Pharma at AAAAI2024
WASHINGTON, Feb. 25, 2024 /PRNewswire/ -- Longbio Pharma (Suzhou) Co., Ltd. (referred to as "Longbio Pharma"), a leading biotech company dedicated to developing innovative protein treatments for allergy, respiratory, dermatology, hematology, ophthalmology, and other autoimmune and rare diseases, ...
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
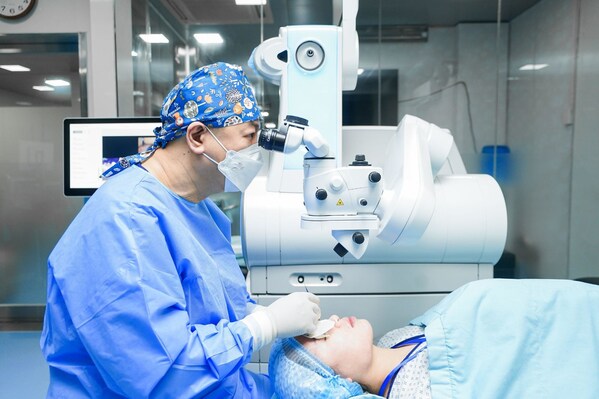
2024-11-11 21:00DND International Eye Hospital: Pioneering SMILE Pro Surgery & Leading the Trend of Refractive Surgery Tourism in Vietnam
[Picked up by 279 media titles]
2024-11-08 20:11