Health
China Medical University Hospital's Breakthrough: Leg Salvage Against Deadly Arterial Disease. Amputation and Death Rates Plunge from 30% to Below 3%
TAICHUNG, Feb. 24, 2024 /PRNewswire/ -- In winter, cardiovascular diseases are more prevalent, which significantly increase the likelihood of arterial blockage. In addition to increasing the possibility of accompanying myocardial infarction or stroke, seniors should be especially cautious about P...
China Pharma Holdings Inc. Announces 1-for-5 Reverse Stock Split
HAIKOU, China, Feb. 23, 2024 /PRNewswire/ -- China Pharma Holdings Inc. ("China Pharma" or the "Company") (NYSE American: CPHI) reported that it expects to implement a 1-for-5 reverse stock split on its common stock effectiveWednesday, March 6, 2024, with trading to begin on a split-adjusted basi...
HeyGears Unveils Automated Case-by-Case Clear Aligner Production Solution
IRVINE, Calif., Feb. 23, 2024 /PRNewswire/ -- HeyGears
Oscotec/ADEL Initiates First-in-Human Dosing in Ph1 of Anti-MTBR Tau Antibody ADEL-Y01 In Alzheimer's Disease
PANGYO, South Korea, Feb. 23, 2024 /PRNewswire/ -- Oscotec Inc. and ADEL Inc. announced the dosing of the first healthy participant in its first-in-human study of ADEL-Y01 for the treatment of Alzheimer's disease (AD). Oscotec and ADEL are jointly developing a novel disease-modifying immunothera...
Immunofoco Announces the Dual Approval of IND Applications by the U.S. FDA and China CDE for the First EpCAM CAR-T Targeted at Advanced Solid Tumors
- The first EpCAM targeted CAR-T product obtained US/CN IND approval. - Acceptable safety profiles and preliminary efficacy were observed in Investigator-Initiated Trial (IIT) clinical studies of IMC001. SHANGHAI, Feb. 22, 2024 /PRNewswire/ -- Immunofoco, a company dedicated to developing cell ...
Jolly Good Revolutionizes Healthcare with Immersive Service for Apple Vision Pro, Premiering at SXSW 2024
A Milestone Entry into Spatial Computing Healthcare BROOKLINE, Mass., Feb. 22, 2024 /PRNewswire/ -- Jolly Good US Inc. proudly presents "JOLLYGOOD+ for Vision Pro," a groundbreaking immersive medical service harnessing the power of spatial computing through Apple Vision Pro technology. This inno...
CATUG and Crystal Bio Establish Strategic Partnership, Launching "CATUG-Crystal" Joint Lab Dedicated to Advanced Nucleic Acid Analytical Services
CAMBRIDGE, Mass. and CRANBURY, N.J., Feb. 22, 2024 /PRNewswire/ -- CATUG Inc. (CATUG) andCrystal Bio, a member of Crystal Pharmatech, announced today a long-term strategic partnership to provide advanced nucleic acid-based drug analytical services. CATUG, a distinguished global entity specializin...
VOCIC Presents its Latest Products at Arab Health 2024 in Dubai
CITY OF INDUSTRY, Calif., Feb. 21, 2024 /PRNewswire/ -- The VOCIC team showcased its latest home medical care and senior care products at Arab Health 2024 in Dubai, held from January 29th to February 1st. The exhibition provided a crucial chance to present VOCIC's innovative healthcare solutions t...
Mabwell Publishes the Phase III Study Results on Its Denosumab Biosimilar (MW032) in the journal JAMA Oncology
SHANGHAI, Feb. 21, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, recently published the phase III study results of denosumab biosimilar (MW032) online in the international top journal of JAMA Oncology. This is the first recorded tria...
WuXi Biologics Recognized as Both Industry and Regional ESG Top-Rated Company by Morningstar Sustainalytics
* Named Top-Rated for fourth consecutive year * Committed to generating long-term value for all stakeholders SHANGHAI, Feb. 21, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), announced it has ...
Zylox-Tonbridge Receives Marketing Approvals for Multiple Products in the UAE
HANGZHOU, China, Feb. 20, 2024 /PRNewswire/ -- Zylox-Tonbridge (2190.HK), a medical device company specializing in peripheral and neurovascular interventions, proudly announces the recent marketing approvals granted by the Ministry of Health and Prevention in theUnited Arab Emirates for five of i...
Ono Enters into a Research Collaboration Agreement with InveniAI to Identify Novel Therapeutic Targets
OSAKA, Japan, Feb. 20, 2024 /PRNewswire/ -- Ono Pharmaceutical Co., Ltd. ( Osaka, Japan; President and CEO: Gyo Sagara; "Ono") today announced that it has entered into a research collaboration agreement with InveniAI® LLC (Guilford, Connecticut, USA; President and CEO: Krishnan Nandabalan; "Inveni...
Hox Therapeutics and Vernalis announce a drug discovery collaboration in oncology
CHENGDU, China, Feb. 20, 2024 /PRNewswire/ -- Hox Therapeutics Ltd ("Hox") a private biotechnology company developing highly targeted cancer therapies and Vernalis (R&D) Ltd ("Vernalis"), a fully owned subsidiary of HitGen Inc., are pleased to announce a collaboration to identify inhibitors again...
FDA Grants Orphan Drug Designation to 9MW3011
SHANGHAI, Feb. 20, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that FDA has granted Orphan Drug Designation (ODD) to 9MW3011 (R&D code in the US: MWTX-003/DISC-3405) for the treatment of patients with polycythemia vera (P...
Mabwell Receives IND Approval from FDA for Novel B7-H3 ADC 7MW3711
SHANGHAI, Feb. 20, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its clinical trial application of B7-H3 targeting ADC (R&D code: 7MW3711) for advanced malignant solid tumor was approved by the U.S. Food and Drug Admi...
WuXi Advanced Therapies Receives FDA Approval to Manufacture Iovance's AMTAGVI™ (lifileucel) for Advanced Melanoma
AMTAGVI is the first and only one-time, individualized T cell therapy to receive U.S. FDA approval fora solid tumor cancer. PHILADELPHIA, Feb. 20, 2024 /PRNewswire/ -- WuXi Advanced Therapies (WuXi ATU), a wholly owned subsidiary of WuXi AppTec, today announced that the U.S. Food and Drug Admini...
Origin Agritech Regains Compliance with Nasdaq Listing Standards
BEIJING, Feb. 20, 2024 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), a leading Chinese agricultural technology company, today announced that it has regained compliance with Nasdaq's market value of listed securities requirement as set forth in Listing Rule 5550(...
Concord Medical Announces Receipt of NYSE Non-Compliance Letter Regarding ADS Trading Price
BEIJING, Feb. 20, 2024 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specialized in cancer treatment, research, education and prevention inChina, today announced that it has received a letter (the "Letter") from t...
Kangpu Biopharmaceuticals to Present Preclinical Efficacy Data of KPG-818 in Crohn's Disease at the 19th Congress of ECCO (IND Application for Phase II in Progress)
HEFEI, China, Feb. 20, 2024 /PRNewswire/ -- Kangpu Biopharmaceuticals, a clinical-stage company dedicated to the discovery and development of novel therapeutics for the treatment of cancer, autoimmune diseases, and inflammation, through targeted protein degradation technology, today announced th...
Neurophth Announces Completion of Patient Enrollment for Opvika® Phase I/II Clinical Trial in the U.S.
WUHAN, China and SAN DIEGO, Feb. 19, 2024 /PRNewswire/ -- Neurophth Therapeutics, Inc. ("Neurophth") announced today thatthe last patient has been enrolled in Phase I/II clinical trial of Opvika® (Esonadogene Imvoparvovec) for the treatment of Leber hereditary optic neuropathy caused byND4 mutati...
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
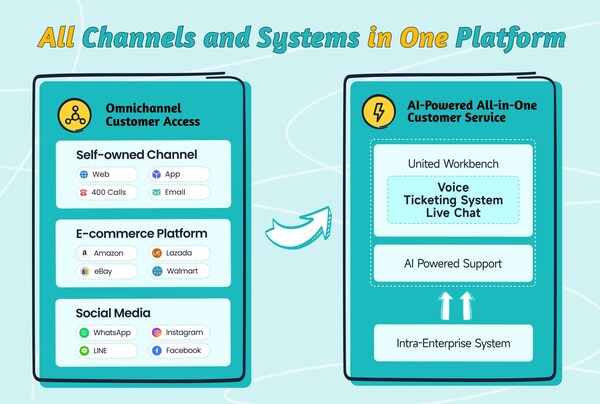
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
2024-11-11 21:00Transformation of Asia's Process Industries To Get A Boost Through Region's First Dedicated Process Technology Show
[Picked up by 279 media titles]
2024-11-12 16:00