 |
AMSTERDAM and ORLANDO, Fla., March 12, 2018 /PRNewswire/ -- Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced health economic results from the DEFINE FLAIR clinical trial comparing cost-effectiveness between instant wave-free ratio (iFR) and fractional flow reserve (FFR) in the guidance of treatment of ischemic heart disease at the American College of Cardiology (ACC) annual meeting in Orlando, Florida, March 10-12, 2018. The study found that an iFR-guided strategy offers a one-year average cost savings of US$896 per patient compared to an FFR-guided strategy, while delivering consistent patient outcomes. iFR is an innovative pressure-derived index unique to Philips, a global leader in image-guided therapy solutions, allowing a simplified hyperemia-free physiological assessment of coronary blockages.
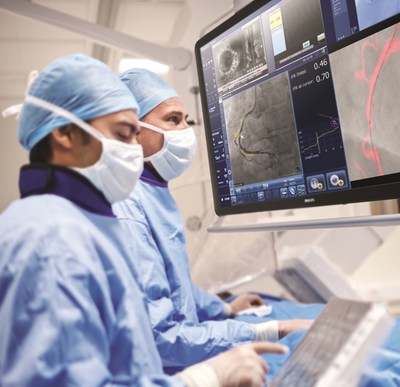
Coronary artery disease is the most common type of heart disease and is the leading cause of death in the U.S. for men and women[1]. Coronary physiology has become routine in planning coronary revascularization, and DEFINE FLAIR compared using iFR and FFR strategies to guide these types of procedures. With an average saving of nearly US$900 per patient per year, the study found that iFR offers a total procedure cost saving of approximately 10 percent per patient over FFR. Additionally, patients treated with the use of an iFR-guided revascularization strategy had fewer coronary artery bypass graft procedures and fewer subsequent revascularizations. Previous data from DEFINE FLAIR released in 2017 found that iFR-guided treatments reduced procedure time by 10 percent versus FFR-guided treatments, while reducing patient discomfort by 90 percent[2]. The pivotal DEFINE FLAIR study continues to illustrate the advantages of iFR and the superior value it delivers to clinicians and hospital administrators.
"The findings from DEFINE FLAIR continue to demonstrate the benefits of iFR, showing that an iFR-guided treatment offers proven outcomes, reduced costs and procedure time, and enhanced patient comfort compared to FFR," said Dr. Manesh Patel, MD, FACC, FSCAI, Chief of the Division of Cardiology and Co-Director of the Duke Heart Center at Duke University School of Medicine. "iFR is not only a faster diagnostic solution, but it also offers the advantage of significantly reduced patient discomfort. By implementing an iFR program at a hospital, this solution can deliver the clinical outcome benefits of physiology-driven PCI, while reducing annual health care costs significantly across the organization."
In the treatment of coronary artery disease, clinicians have been using FFR in addition to angiographic images to assess the physiology of a suspected blockage in coronary arteries in order to identify the appropriate therapy for each patient. iFR is a next-generation physiologic measurement that uses the same pressure guide wires and equipment as FFR, but avoids the administration of hyperemic agents, which are required with FFR, to the patients.
"The DEFINE FLAIR study has provided further clinical validation of iFR and how it is improving the lives of patients and physicians," said Christopher Barys, Business Leader of Philips Image Guided Therapy Devices. "An iFR-guided strategy has now been shown to improve patient outcomes and reduce costs for the treatment of coronary artery disease in comparison to an FFR-guided strategy. This is a significant step in our journey to help clinicians decide, guide, treat and confirm the right therapies for their patients, while reducing costs."
DEFINE FLAIR is a randomized, controlled, single-blinded comparison of clinical outcomes and cost efficiencies of iFR and FFR-guided interventions of 2,492 patients in 49 centers across Europe, Asia, North America, and Africa. The study conducted its comparison of iFR versus FFR using pressure guide wires and equipment from Philips. Since the introduction of the hyperemia-free iFR modality in 2013, iFR has been studied in nearly 15,000 patients and is used in more than 4,100 catheterization labs across the world.
See Philips' calendar of events for ACC, as well as general information about the company's presence at the show, online here. Visit the Philips booth (#2811) to experience its innovative cardiology portfolio that delivers integrated solutions to the full health continuum. Follow the #ACC18 conversation on @PhilipsLiveFrom throughout the event.
[1] Coronary Artery Disease, NIH, 2015
[2] Davies JE, et al., DEFINE-FLAIR: A Multi- Centre, Prospective, International, Randomized, Blinded Comparison of Clinical Outcomes and Cost Efficiencies of iFR and FFR Decision-Making for Physiological Guided Coronary Revascularization. New England Journal of Medicine, epub March 18, 2017
For further information, please contact:
Mark Groves
Philips Group Press Office
Tel: +31 631 639 916
E-mail: Mark.Groves@Philips.com
Fabienne van der Feer
Philips Image Guided Therapy
Tel: +31 6-22698001
E-mail: Fabienne.van.der.Feer@philips.com
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips' health technology portfolio generated 2017 sales of EUR 17.8 billion and employs approximately 74,000 employees with sales and services in more than 100 countries. News about Philips can be found at: www.philips.com/newscenter.
Photo - https://mma.prnewswire.com/media/652217/Royal_Philips_iFR___FFR.jpg
Logo - https://mma.prnewswire.com/media/556483/Philips_Logo.jpg