SUZHOU, China, June 18, 2019 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops and commercializes high quality medicines, today announced that the clinical results of CT103A, the potential best-in-class therapy of fully-human BCMA CAR-T co-developed by Innovent and Nanjing IASO Biotherapeutics (IASO BIO), was presented by oral presentation and poster at two of the most prestigious clinical meetings in the worlds of hematology and oncology, the 24th Congress of the European Hematological Society (EHA) [Abstract #S827; Saturday, June 15, 12:35 PM - 12:45 PM CEST] and the American Society of Clinical Oncology (ASCO) Annual Meeting 2019 in Chicago Illinois [Abstract #8013; Tuesday, June 4, CDT]. The IIT study was conducted at Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology.
CT103A is an anti-BCMA CAR-T co-developed by Innovent and IASO BIO for the treatment of Relapsed/Refractory Multiple Myeloma (RRMM). The data of CT103A presented at both conferences show an impressive efficacy results, persistence and safety profile and an objective response rate (ORR) of 100%. The data are especially encouraging for patients who relapsed from a prior CAR-T treatment with mouse-based antibody, thus providing a viable option for this group of tough-to-treat patients.
Multiple myeloma is a malignant hematologic cancer with abnormal proliferation of clonal plasma cells, which has no medical cure so far. In many countries, myeloma is the second most common blood cancer. The American Cancer Society estimates that in the United States (U.S.), about 32,110 new cases will be diagnosed this year. In Europe, more than 48,200 people were diagnosed with multiple myeloma in 2018. Among them, 40 percent of patients are diagnosed with moderate or high-risk multiple myeloma, and their median survival is less than five years.
As of the data cutoff (22 May 2019), the objective response rate (ORR) was 100% (CR-64%, VGPR-36%) with strong persistence and high expansion of the CAR-T in-vivo. All patients (100%) experienced CRS. The onset of CRS occurred within 2-5 days (median-2.6) and resolved within 14 days. Mostly grade 1 and 2, at the lowest dosage levels, CRS was routinely managed with Tocilizumab and steroids. Interestingly, the 11 patient study included 4 patients having previously relapsed from a prior CAR-T therapy, a murine anti-BCMA CAR-T.

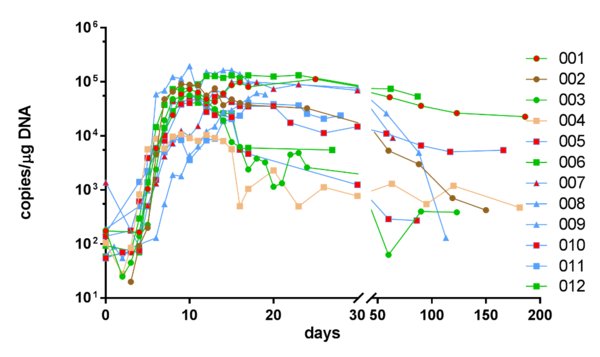
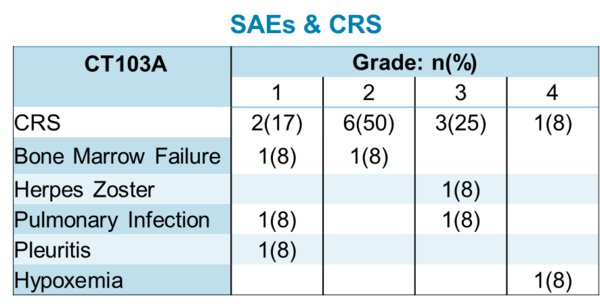
"RRMM is associated with a poor prognosis," said Dr. Chunrui Li of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology. "Many patients who receive CAR-T treatments have had their disease recurrence, and with a non-human scFv, re-treatment may not be an option due to immunogenicity. With a fully-human BCMA scFv, CT103A provides an effective option for these patients. This data suggests they should not be excluded from the benefit of future trials."
About RRMM
For newly treated patients with multiple myeloma, the common first-line treatment drugs include proteasome inhibitors, immunoregulatory drugs and alkane agents. For most patients, the commonly used first-line treatment can stabilize the patient's condition for 3-5 years, but a small number of patients show primary drug resistance at the time of initial treatment, and the disease cannot be effectively controlled. Relapse patients are patients who have reappeared after complete remission of the disease. Refractory patients are patients with primary drug resistance or the patients who have finished with first-line treatment do not achieve remission, or the patients whose disease progress within 60 days after achieving minimal response. For the majority of patients with effective treatment, they will inevitably enter the stage of relapse and refractory after 3-5 years of disease stabilization. For these patients, the overall effective rate of existing second-line treatment is about 40% to 70%, with short remission time.
About CT103A
CT103A is an innovative therapy co-developed by IASO BIO and Innovent. Previous studies indicate patients with RRMM who received high-dose BCMA-targeting CAR-T cells may achieve better remission but have worse adverse events. Moreover, once the disease progresses again, the re-infusion of CAR-T cells is not effective. To solve this dilemma, CT103A has been developed, a lentiviral vector containing a CAR structure with a fully-human scFv, CD8a hinger and transmembrane, 4-1BB co-stimulatory and CD3z activation domains. Based on strict selection and screening, utilizing a proprietary in-house optimization platform, the construct of the CT103A CAR-T is potent and persistent.
About Nanjing IASO Biotherapeutics
Founded in 2017, IASO BIO is a clinical stage biotechnology company advancing the development of innovative therapies for cancer. IASO BIO stands out in fierce competition through innovation, a world class facility, and an internationally renowned clinical research team. IASO BIO is dedicated to curing cancer using engineered autologous/allogenic T cell therapies designed to enhance the immune system's ability to recognize and eradicate cancer cells. Currently, IASO BIO is developing over 10 high-potential high-end biopharmaceutical products, targeting hematological tumors, solid tumors and virus associated tumors. For more information, please visit: www.iasobio.com.
About Innovent
Inspired by the spirit of "Start with Integrity, Succeed through Action," Innovent's mission is to develop and commercialize high quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to developing, manufacturing and commercializing high quality innovative medicines for the treatment of oncology, autoimmunity and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since it was founded, Innovent has developed a fully-integrated platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities. Leveraging the platform, the company has built up a robust pipeline of 20 innovative assets in the fields of oncology, ophthalmology, autoimmunity, and cardiovascular diseases. Fourteen assets have entered into clinical development, four have entered Phase 3 clinical trials, three monoclonal antibodies have their New Drug Application (NDA) under review and two of them have been granted with priority review status, and one, Tyvyt® (sintilimab injection), is now approved for relapsed or refractory classical Hodgkin's lymphoma (r/r cHL).
Innovent has built an international team of advanced talents in high-end biological drug development and commercialization, including many overseas experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, Hanmi and other international pharmaceutical companies. Innovent strives to work with all relevant parties to help advance China's biopharmaceutical industry, improve drug availability to ordinary people and enhance the quality of the patients' lives. For more information, please visit:www.innoventbio.com.
Photo - https://photos.prnasia.com/prnh/20190617/2499313-1-a
Photo - https://photos.prnasia.com/prnh/20190617/2499313-1-b
Photo - https://photos.prnasia.com/prnh/20190617/2499313-1-c