 |
IRVINE, Calif., March 11, 2021 /PRNewswire/ -- Zymo Research announced today the U.S. Food and Drug Administration (FDA) has cleared its DNA/RNA Shield™ Collection Tube as a Class II medical device. The FDA's 510(k) clearance allows the product to be used as an In-vitro Diagnostic (IVD) device for COVID-19 testing.
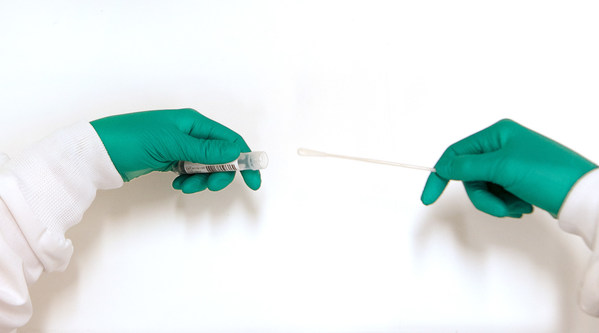
Specifically granted for COVID-19 testing, the DNA/RNA Shield™ Collection device is the first FDA-cleared technology that inactivates the virus and preserves the SARS-CoV-2 RNA. The SARS-CoV-2 virus is effectively inactivated, which allows the sample to be safely handled, transported, and stored. This provides for the safety of frontline healthcare and laboratory workers. The viral RNA is stabilized at ambient temperature for prolonged periods for robust analysis via downstream RT-PCR.
"DNA/RNA Shield had a proven track record in various infectious disease applications prior to the current pandemic, facilitating its rapid adoption and deployment in the early stages of the COVID outbreak," said Dr. Marc Van Eden, Vice President of Business Development at Zymo Research. "The 510(k) is the result of the FDA's active collaboration with Zymo Research in bringing this technology to the forefront of current testing and future surveillance efforts."
The product consists of a tube filled with Zymo Research's proprietary DNA/RNA Shield™ transport medium. The transport medium ensures the stability of the SARS-CoV-2 RNA during sample transportation and storage for up to 28 days at ambient temperatures. The DNA/RNA Shield™ transport medium may be kitted with a swab, sputum collection kit, or as a tube alone.
The technology is compatible with upper and lower respiratory human specimens suspected of containing SARS-CoV-2. Specimens collected and stored in a DNA/RNA Shield™ Collection Tube are suitable for use with appropriate molecular diagnostic tests.
For more information visit Zymo Research's website or contact them via email at covid19requests@zymoresearch.com.
About Zymo Research Corp.
Zymo Research is a privately owned company that has been serving the scientific and diagnostics community with state-of-the-art molecular biology tools since 1994. "The Beauty of Science is to Make Things Simple" is their motto, which is reflected in all of their products, from epigenetics to DNA/RNA purification technologies. Historically recognized as the leader in epigenetics, Zymo Research is breaking boundaries with novel solutions for sample collection, microbiomic measurements, diagnostic devices, and NGS technologies that are high quality and simple to use. Follow Zymo Research on Facebook, LinkedIn, Twitter, and Instagram.
For more information about Zymo Research's COVID-19 products, check out the following links:
Photo - https://mma.prnasia.com/media2/1454300/510k_shield_fda_certified_pr_2000x1111px_v2.jpg?p=medium600
Logo - https://mma.prnasia.com/media2/364743/Zymo_Research_Corp_Logo.jpg?p=medium600