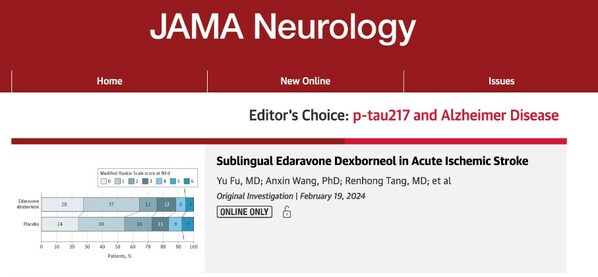
Findings of a Phase III Clinical Study of Sanbexin® Sublingual Tablets Published in JAMA
NANJING, China, Feb. 21, 2024 /PRNewswire/ -- On February 19, 2024, the Journal of American Medical Association•Neurology (JAMA NEUROLOGY, IF: 29.0) published online the key findings of a phase III clinical study (NCT04950920) (the "TASTE-SL Study") investigating Sanbexin® (a combination of edara...
Simcere and Connect Enter into an exclusive license and collaboration agreement for the innovative autoimmune drug Rademikibart
NANJING, China, Nov. 23, 2023 /PRNewswire/ -- On November 21, 2023, Simcere Pharmaceutical Group Limited ("Simcere") entered into an exclusive license and collaboration agreement (the "Agreement") with Connect Biopharma HongKong Limited ("Connect") in relation to Rademikibart, an innovative IL...
Four innovative drugs launched in three years, HKEX -Listed Simcere Pharmaceutical Group is on the Fast Track Toward Building an Innovation-Driven Global Pharmaceutical Company
-The revenue from innovative drugs accounted for more than 65% of total revenue -Simcere's total revenue and the revenue from innovative drugs hit record highs. NANJING, China, April 24, 2023 /PRNewswire/ -- On Apr. 18, Simcere Pharmaceutical (2096.HK) disclosed the latest progress on the compa...
Simcere Zaiming Announces Clinical Collaboration to Evaluate SIM0235, a TNFR2 Monoclonal Antibody, in Combination with KEYTRUDA® (pembrolizumab) in a Phase 1 Trial in Advanced Solid Tumors and Cutaneous T-cell Lymphoma
NANJING, China, March 14, 2023 /PRNewswire/ -- Simcere Pharmaceutical Group Limited (2096.HK) (Simcere), an innovative global biopharmaceutical company, announced today that Simcere Zaiming, an innovative oncology pharmaceutical company of Simcere has entered into a clinical collaboration agreeme...
Simcere and Almirall Enter into a Licensing Agreement for IL-2 mu-Fc
* Simcere will receive a $15 million upfront payment, and up to $492 million in development and commercial milestone payments considering successful achievements in several indications, with an important part as sales milestones, as well as up to low double-digit tiered royalties based upon fu...
Simcere Pharmaceutical Announces Financial Results for 2022 H1: 27% Year-Over-Year Revenue Growth, with Innovative Drugs Accounting for 65.4% of Total Revenue
NANJING, China, Sept. 5, 2022 /PRNewswire/ -- Simcere Pharmaceutical Group Limited (2096.HK) announced its financial results for the first half of 2022. As ofJune 30, Simcere recorded operating revenue of RMB 2.7 billion for the first half of the year, with a year-over-year growth of 27.3%. The e...
Tamas Oravecz Ph.D. named SVP, CSO of the U.S. of Simcere Pharmaceutical Group
NANJING, China, Sept. 5, 2022 /PRNewswire/ -- Simcere Pharmaceutical Group announces today the appointment ofTamas Oravecz, Ph.D. as Senior Vice President, Chief Scientific Officer of the Simcere U.S. Tamas will be responsible for providing strategic leadership to our drug discovery efforts in A...