Antengene Announces Commercial Availability of XPOVIO® (Selinexor), for the Treatment of Relapsed/Refractory Multiple Myeloma, Prescribed for the First Time Across Mainland China
-Distribution channels in place to streamline/facilitate patient access. -XPOVIO® will be available in 600 hospitals and 105 DTPs (across China includingBeijing, Shanghai, Guangdong, Jiangsu, Zhejiang, Henan, and Shandong). SHANGHAI and HONG KONG, May 16, 2022 /PRNewswire/ -- Antengene Corporatio...
Antengene to Participate in Three Upcoming Investor Conferences in May/June
CICC Healthcare Industry Forum 2022: May 9th to 12th BTIG Virtual China Biotech Day: June 1st Citi's 3rd Pan-Asia Regional Investor Conference 2022: June 8th to June 10th SHANGHAI and HONG KONG, May 6, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading inno...
Antengene Announces Latest Clinical Trial Data of ATG-008 (onatasertib) to be presented in the upcoming 2022 American Society of Clinical Oncology Annual Meeting
SHANGHAI and HONG KONG, April 29, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hematology and onc...
Antengene Announces Submission to the Human Research Ethics Committee in Australia for a Phase I Trial of ATG-018
* Developed in-house by internal R&D Team at Antengene, ATG-018 is an orally-available, small molecule ATR inhibitor that targets DNA Damage Response (DDR) pathways. * The Phase I study will evaluate the safety, pharmacology and preliminary efficacy of ATG-018 in patients with advanced solid ...
Antengene Announces Publication of Five Posters at the 2022 American Association for Cancer Research (AACR) Annual Meeting
The company will present the preclinical data of five novel drug candidates at AACR 2022 (onApril 8-13). Focus on ATG-037, ATG-018, ATG-022, ATG-012 and ATG-008. SHANGHAI and HONG KONG, April 11, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovativ...
Antengene's Pivotal "MARCH" Study to Evaluate Selinexor (ATG-010) in Relapsed or Refractory Multiple Myeloma Published in BMC Medicine
- Results showed an overall response rate (ORR) of 29.3% for all treated patients. - Consistent responses across all risk groups, including patients who received prior "triple-class" and CAR-T therapies or having cytogenetic abnormalities. - Selinexor is on track for a Q2:22 launch in China. SHAN...
Antengene Announces IND Approval in China for a Phase II Study of Eltanexor (ATG-016) in Patients with High-Risk Myelodysplastic Syndromes
* This study is the third ATG-016 study to be conducted in China. * Study highlights Antengene's robust SINE programs. SHANGHAI and HONG KONG, March 30, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutic...
Antengene Announces 2021 Full Year Financial Results and Provides Corporate Updates
SHANGHAI and HONG KONG, March 18, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for canc...
Antengene Announces IND Approval in China for the Phase I Study of ATG-101 (PD-L1/4-1BB Bispecific Antibody) for the Treatment of Solid Tumors and Non-Hodgkin Lymphoma
- The novel bispecific antibody ATG-101 is Antengene's first in house developed molecule with global rights. - This approval in China marks the third regulatory clearance granted to ATG-101 globally. - Upon receiving clearances in Australia and the U.S. in 2021, the Phase I study of ATG-101 ...
Antengene Announces Five Upcoming Presentations at the 2022 American Association for Cancer Research Annual Meeting
* Posters Showcase Early-Stage Clinical and Preclinical Pipeline. * Focus on ATG-037, ATG-018, ATG-022, ATG-012 and ATG-008. SHANGHAI and HONG KONG, March 9, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, global biopharmaceutical company ...
Antengene Announces XPOVIO® Approved by the TGA in Australia for the Treatment of Relapsed and/or Refractory Multiple Myeloma and Triple Class-Refractory Multiple Myeloma
- XPOVIO®, in combination with bortezomib and dexamethasone (XBd), gives patients with relapsed and/or refractory multiple myeloma, a new treatment option. - XPOVIO®, in combination with dexamethasone (Xd), gives patients with triple class refractory multiple myeloma, a new treatment option. ...
Antengene Announces XPOVIO® Regulatory Approval in Singapore for the Treatment of Relapsed and/or Refractory Multiple Myeloma and Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Three Indications
SHANGHAI and HONG KONG, March 2, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for cancer and other life...
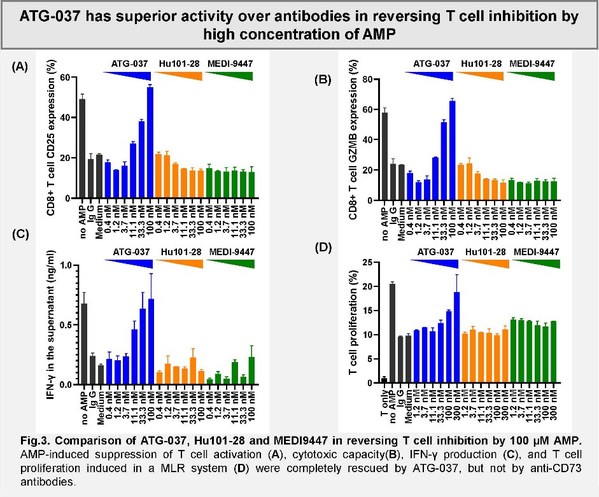
Antengene Announces HREC Approval in Australia for the Phase I Trial of the Small Molecule CD73 Inhibitor ATG-037
SHANGHAI and HONG KONG, Feb. 7, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hematology and o...
Antengene to Present at the 40th Annual J.P. Morgan Healthcare Conference
SHANGHAI and HONG KONG, Jan. 5, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hematology and oncol...
Antengene Announces NDA Approval by NMPA for XPOVIO®, China's First XPO1 inhibitor, for the Treatment of Adults with Relapsed or Refractory Multiple Myeloma
SHANGHAI and HONG KONG, Dec. 17, 2021 /PRNewswire/ -- Antengene Corporation Limited (the "Company" or "Antengene") is pleased to announce thatATG-010 (selinexor, brand name:XPOVIO®), a first-in-class, oral Selective Inhibitor of Nuclear Export (SINE) compound has received conditional approval for...
Antengene and XtalPi Announce AI-Driven R&D Collaboration
SHANGHAI and HONG KONG, Dec. 16, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for cancer and other life...