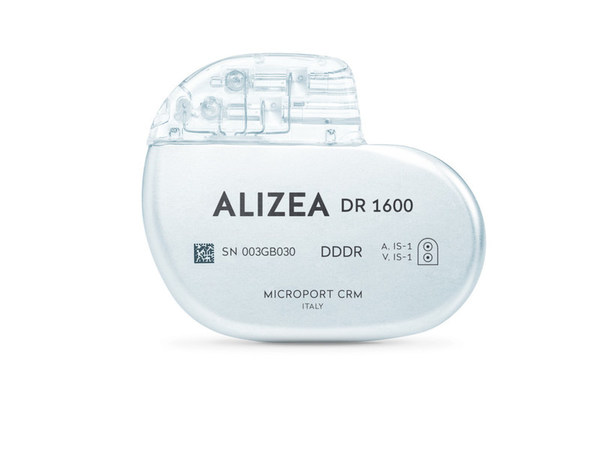
MicroPort CRM Receives Approval in Japan for Alizea Bluetooth Pacemaker
CLAMART, France, Jan. 17, 2022 /PRNewswire/ -- MicroPort CRM, a pioneering company in the field of Cardiac Rhythm Management, headquartered inFrance, recently received PMDA Japanese regulation agency approval for its latest range of implantable pacemakers, Alizea. The devices are equipped with Bl...
MicroPort® Cardiac Rhythm Management Business Announces US$150 Million Series C Investment
SHANGHAI, July 27, 2021 /PRNewswire/ -- MicroPort Scientific Corporation (" MicroPort®") announced that MicroPort Cardiac Rhythm Management Limited (" MicroPort® CRM"), which is MicroPort®'s subsidiary focused on developing and commercializing implantable pacemaker and defibrillator devices and rel...
Firehawk Clinical Data From The TARGET AC European Clinical Trial Has Been Accepted For Publication In The Prestigious Medical Journal The Lancet
SHANGHAI, Sept. 4, 2018 /PRNewswire/ -- MicroPort Scientific Corporation ("MicroPort®", HK:0853) announced today that the clinical trial results for its Firehawk® Rapamycin Target Eluting Coronary Stent System ("Firehawk®") which conducted a multi-center, randomized controlled trial called TARGET...
MicroPort(TM)'s Firehawk(TM) Drug Eluting Stent Achieves Primary Endpoint and Shows Very Promising Results From TARGET All-Comers Clinical Trial
PARIS, May 23, 2018 /PRNewswire/ -- Today MicroPort Scientific Corporation (HKSE: 0853) ("MicroPort™") announced primary endpoint data at 12 months and QCA angiography data at 13 months from its TARGET All-Comers (TARGET AC) trial. The results of the TARGET AC trial demonstrated that vessels trea...