WuXi Biologics Successfully Completes First 1,6000L Manufacturing Run in Ireland
- First manufacturing run successful for MFG7 facility at the Ireland site - Largest manufacturing scale to date achieved by combining four 4,000-liter single-use bioreactors DUNDALK, Ireland, Jan. 31, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Researc...
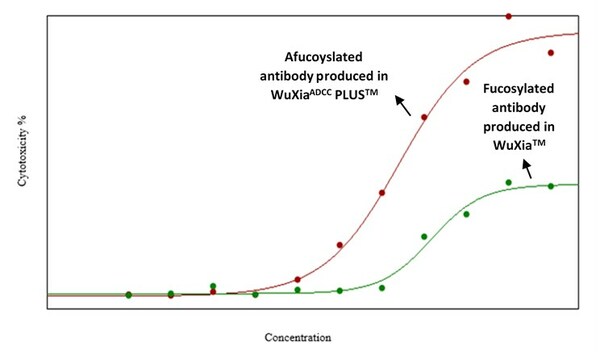
WuXi Biologics Launches WuXia ADCC PLUS™ for the Development and Manufacturing of Afucosylated Antibodies that Elicit Enhanced ADCC Effect
- WuXiaADCC PLUSTM is designed to meet the market need for improved antibody therapeutic efficacy by producing afucosylated antibodies with the ability to increase antibody-dependent cell-mediated cytotoxicity (ADCC), providing diverse bioprocessing solutions for global clients - WuXiaADCC PLU...
WuXi Biologics Granted U.S. Patent for Proprietary Bispecific Antibody Technology Platform WuXiBody™
SHANGHAI, Jan. 16, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that a patent for WuXiBodyTM – a proprietary, highly flexible engineering platform that greatly enhances the deve...
WuXi Biologics to Increase Manufacturing Capacity in Massachusetts
* The added capacity will further enhance WuXi Biologics' commercial manufacturing capabilities in the U.S. * The site is expected to employ 250 people when fully operational. WORCESTER, Mass., Jan. 8, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Resea...
WuXi Biologics Named to 2023 Dow Jones Sustainability World Index
* Identified as a global sustainability leader by S&P Global * Committed to generating long-term value for all stakeholders SHANGHAI, Dec. 12, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), an...
WuXi Biologics Awarded Highest Platinum Medal by EcoVadis Sustainability Rating
* Ranked in top 1% of the over 100,000 companies evaluated * Recognized for outstanding performance across all areas: Environment, Labor & Human Rights, Ethics, and Sustainable Procurement * Seen as a trusted partner by global clients for its strong sustainability commitment SHANGHAI, Dec. 8...
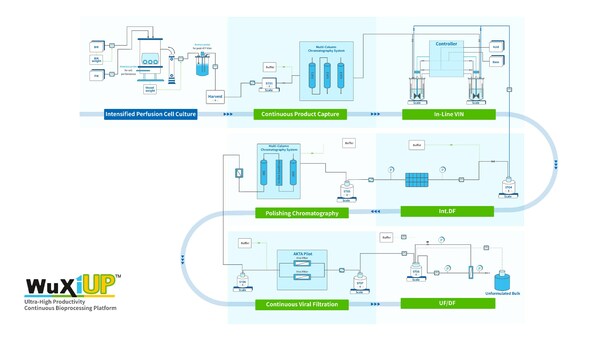
WuXi Biologics Successfully Implemented a Fully Integrated Continuous Process with a Breakthrough Productivity of ~6 g/L/day at Pilot Scale
* By leveraging WuXiUPTM, WuXi Biologics has completed its first end-to-end continuous drug substance (DS) manufacturing from perfused cell culture to final UF/DF pool at pilot scale * This end-to-end continuous bioprocess, which is in place for WuXi Biologics global manufacturing network, is...
WuXi Biologics Receives AAA MSCI ESG Rating
SHANGHAI, Nov. 7, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), announced it has received an AAA rating from Morgan Stanley Capital International (MSCI) ESG Ratings, the highest rating for compan...
WuXi Biologics Launches New High-Productivity Bioprocessing Platform WuXiUI™ for Cost-Effective Commercial Manufacturing and Desirable Product Quality
* WuXiUI™ ultra-intensified fed-batch platform offers a new process option for maximum flexibility to meet various clients' needs in biologics development and manufacture. The platform can significantly improve productivity and quality, and reduce cost-of-goods (COGS) in commercial manufacturin...
WuXi Biologics Congratulates Amicus Therapeutics on U.S. FDA Approval for New Treatment for Pompe Disease
WUXI, China, Oct. 1, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), congratulates its strategic partner, Amicus Therapeutics ("Amicus") (Nasdaq: FOLD), on receiving U.S. FDA's approval for Pombili...
WuXi Biologics and Boostimmune Sign MOU for Exclusive Research and Discovery Services
SHANGHAI, Aug. 10, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), and Boostimmune, a biotech company dedicated to developing next-generation anti-cancer therapies modulating the tumor immune micr...
WuXi Biologics to Increase Manufacturing Capacity in Germany
- In response to global clients' increasing demand for contract manufacturing services, WuXi Biologics is expanding its capacity for drug substance and drug product inGermany - The investment promotes the creation of additional high-skilled jobs - As part of WuXi Biologics' global manufacturi...
WuXi Biologics Receives 2023 ISPE Facility of the Year Award for Operations
DUNDALK, Ireland, May 22, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269. HK), a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), today announced thatthe International Society for Pharmaceutical Engineering (ISPE) has awarded the company a 2023 Facili...
WuXi Biologics Announces Manufacturing Partnership with InflaRx to Advance Gohibic for the Treatment of Certain Critically Ill COVID-19 patients
* WuXi Biologics is the manufacturing partner of InflaRx for Gohibic (vilobelimab), a first-in-class monoclonal anti-C5a antibody. * InflaRx received emergency use authorization (EUA) from the FDA for Gohibic (vilobelimab) for the treatment of certain critically ill COVID-19 patients in April ...
WuXi Biologics Releases 2022 ESG Report Detailing Its Continued Commitment to Sustainability
* The Report outlines new goals of net-zero GHG emissions across all operations by 2050 and a 10% reduction of waste intensity by 2027 compared with 2022. * The Company makes remarkable progress on environmental goals, achieving its water consumption intensity-reduction target in 2022, well a...
WuXi Biologics Congratulates Amicus Therapeutics on European Commission Approval for Pombiliti™ in Patients with Late-Onset Pompe Disease
WUXI, China, March 28, 2023 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), congratulates its strategic partner Amicus Therapeutics ("Amicus") (Nasdaq: FOLD) on receiving European Commission's approval ...
WuXi Biologics Reports Remarkable 2022 Annual Results
Revenue Increased by 48.4% Y-o-Y to RMB15,268.7 Million Gross Profit Increased by 39.2% Y-o-Y to RMB6,724.0 Million Adjusted Net Profit Rose by 47.1% to RMB5,053.9 Million Non-COVID Revenue Achieved 63% Y-o-Y Growth, Strong Momentum Continues into 2023 and Beyond "R" in CRDMO Business Model A...
WuXi Biologics and GSK Enter into License Agreement on Multiple Novel Bi- & Multi-specific T Cell Engagers
* WuXi Biologics will provide an exclusive license to GSK for one preclinical bi-specific T cell engaging (TCE) antibody and the option of three additional bi-/multi-specific TCE antibodies developed using WuXi Biologics' proprietary technology platforms * WuXi Biologics will receive an upfro...
WuXi Biologics (Shanghai) Co., Ltd. Removed from the U.S. Commerce Department's Unverified List
SHANGHAI, Dec. 15, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio", 2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), announced that its subsidiary WuXi Biologics (Shanghai) Co., Ltd. has been removed from the Unverified List (UVL) by the U.S. Depar...
WuXi Biologics Provides Corporate Updates
SHANGHAI, Oct. 27, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269. HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today updated its recent corporate progress. Recent Business Milestone Despite the global dynamic situation and funding challeng...