Henlius Forecasts Profit in 1H 2023
Spurring a Seamless R&D, Manufacturing, and Commercialisation Positive Cycle SHANGHAI, July 3, 2023 /PRNewswire/ -- Henlius (2696.HK) released a positive profit forecast. Based on the preliminary assessment of the unaudited comprehensive management accounts for the six months endedJune 30, 2023, ...
Henlius Achieved Steep Revenue Growth in Q1 2023, Core Products Sales Surged
SHANGHAI, April 17, 2023 /PRNewswire/ -- As a global innovative biopharmaceutical company, Henlius is committed to offering high-quality, affordable and innovative biopharmaceuticals to patients worldwide with 5 products launched inChina. Leveraging the differentiated competitive edges of its pr...
Henlius 2022 Annual Results: Significant Achievements in Commercialisation, Surged to RMB3.2 Billion in Revenues
SHANGHAI, April 1, 2023 /PRNewswire/ -- Henlius (2696.HK) announced its 2022 annual results. In 2022, Henlius' revenue reached aboutRMB3.2147 billion, representing an increase of 91.1% YoY, primarily due to sales revenue and licensing revenue generated by the successive commercialisation of vario...
EMA Validates Marketing Authorization Application for Henlius' HANSIZHUANG (Serplulimab)
* HANSIZHUANG (serplulimab) is the first anti-PD-1 mAb for the first-line treatment of small cell lung cancer (SCLC) * The EC and the FDA previously granted Orphan Drug Designations (ODDs) for HANSIZHUANG in SCLC * HANSIZHUANG is approved in China for microsatellite instability-high (MSI-H)...
Henlius Announces U.S. FDA Acceptance of Biologics License Application for Proposed Biosimilar Trastuzumab HLX02
* The first Chinese biosimilar approved in both China and the EU, and potentially to be approved in the U.S. - * HLX02 (trastuzumab for injection, trade name in China: HANQUYOU; trade name inEurope: Zercepac®; trade names in Australia: Tuzucip® and Trastucip®) has been approved in more than 3...
Henlius' Novel Anti-PD-1 mAb HANSIZHUANG (Serplulimab) Approved for the Treatment of ES-SCLC
* World's first anti-PD-1 mAb for the first-line treatment of SCLC * Making a new record with the median OS of 15.8 months in an international, multi-center, phase 3 clinical trial * Granted orphan drug designations by the FDA and the EC, paving the way for international commercialization SH...
Fosun Pharma and Henlius Entered into an Exclusive License Agreement for Serplulimab in the US
PRINCETON, N.J., Jan. 7, 2023 /PRNewswire/ -- Fosun Pharma (600196.SH, 02196.HK) has recently entered into an exclusive license agreement with Shanghai Henlius Biotech, Inc. (2696.HK) for the commercialisation of Henlius independently developed anti-PD-1 monoclonal antibody (mAb) serplulimab inth...
First Patient dosed in a Head-to-Head First-Line Bridging Study of HANSIZHUANG for ES-SCLC treatment in the US
SHANGHAI, Nov. 30, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the first patient was dosed in NCT05468489, a bridging head-to-head trial inthe United States comparing HANSIZHUANG (serplulimab), an innovative anti-PD-1 monoclonal antibody (mAb) independently develo...
Henlius' Novel Anti-PD-1 mAb HANSIZHUANG (Serplulimab) Receives NMPA Approval for the Treatment of sqNSCLC
SHANGHAI, Nov. 1, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696. HK) announced that its first self-developed innovative anti-PD-1 monoclonal antibody (mAb) HANSIZHUANG (generic name: serplulimab injection), in combination with carboplatin and albumin-bound paclitaxel for the first-lin...
ASTRUM-005: The first immunotherapy clinical study of SCLC published in JAMA, one of the top medical journals in the world
* ASTRUM-005 is the first study to demonstrate that PD-1 inhibitors plus chemotherapy can improve survival for patients with ES-SCLC. * In ASTRUM-005, serplulimab combined with chemotherapy achieved by far the longest OS in first-line immunotherapy for ES-SCLC. Compared with chemotherapy, the...
Henlius 2022 H1 Results: Sharpen all-round edges, advance in evolution to Biopharma
SHANGHAI, Aug. 18, 2022 /PRNewswire/ -- Henlius (2696.HK) announced its 2022 interim results. As a global innovative biopharmaceutical company, Henlius is committed to offering high-quality, affordable and innovative biopharmaceuticals to patients worldwide with 5 products launched inChina, 1 in ...
Henlius HANQUYOU Received TGA Approval in Australia
SHANGHAI, July 26, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the company's business partner Cipla Limited ("Cipla") has received the relevant registration certificates from the Therapeutic Goods Administration ofAustralia ("TGA") for the approval of Henlius' sel...
Palleon Pharmaceuticals and Henlius Enter into Strategic Collaboration to Develop Bifunctional Sialidase Therapies
* Palleon and Henlius to co-develop Bifunctional HER2-Sialidase and a second bifunctional sialidase to be jointly designed - * Henlius received exclusive license for two products in China (including Hong Kong, Macau, and Taiwan); Palleon retains all other global rights - * Palleon receives u...
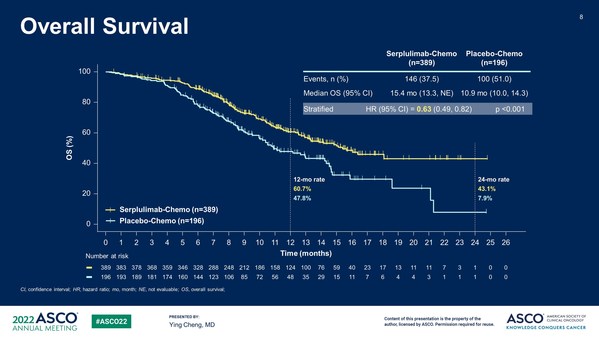
ASTRUM-005: Henlius Released Phase 3 Study Results for the First-line Treatment of Small Cell Lung Cancer of Serplulimab at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that an international randomized phase 3 study (ASTRUM-005) of HANSIZHUANG (serplulimab), an anti-PD-1 mAb independently developed by Henlius, as first-line treatment for extensive-stage small-cell lung can...
The NDA of Henlius Novel Anti-PD-1 mAb Serplulimab for the First-Line Treatment of Small Cell Lung Cancer Accepted by NMPA
SHANGHAI, April 11, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the New Drug Application (NDA) of HANSIZHUANG (serplulimab), a novel anti-PD-1 monoclonal antibody (mAb) independently developed by the company, in combination with chemotherapy for the first-line tr...
Henlius' Serplulimab Granted Orphan-Drug Designation in the United States for Small Cell Lung Cancer
SHANGHAI, April 7, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the United States Food and Drug Administration (FDA) has granted Orphan-Drug Designation (ODD) for HANSIZHUANG (serplulimab) for the treatment of small cell lung cancer (SCLC). This is the first such d...
Henlius Receives NMPA Approval for its First Innovative Monoclonal Antibody HANSIZHUANG
SHANGHAI, March 25, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that its first self-developed innovative PD-1 inhibitor HANSIZHUANG (generic name: serplulimab injection) has been approved by the National Medical Products Administration (NMPA)for the treatment of adult ...
Henlius 2021 Annual Results: New Record in Performance, Evolving to Biopharma
SHANGHAI, March 17, 2022 /PRNewswire/ -- Henlius (2696.HK) announced its annual results for the year endedDecember 31st, 2021, sharing the company's recent noteworthy progress and achievements. As a global innovative biopharmaceutical company, Henlius is committed to offering high-quality, afford...
Henlius 2021 Annual Results: New Record in Performance, Evolving to Biopharma
SHANGHAI, March 16, 2022 /PRNewswire/ -- Henlius (2696.HK) announced its annual results for the year endedDecember 31st, 2021, sharing the company's recent noteworthy progress and achievements. As a global innovative biopharmaceutical company, Henlius is committed to offering high-quality, afford...
Henlius' anti-PD-1 mAb MRCT achieved 15.38 months OS in first-line treatment of SCLC, reducing the risk of death by 38% of the overall population
* The ASTRUM-005 states that serplulimab combined with carboplatin-etoposide prolonged median OS in both the overall population and the Asian subgroup, the median overall survival (OS) in the serplulimab and placebo groups were 15.38 and 11.10 months, respectively, reducing risk of death by 38%...