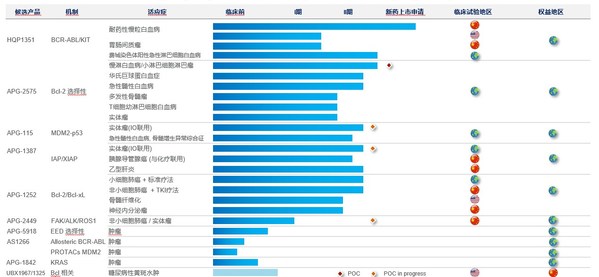
Ascentage Pharma Releases Preclinical Data of Its Core Drug Candidates at AACR Annual Meeting 2021, with Results Signifying the Potential of Multiple Combination Therapies in Cancer
SUZHOU, China and ROCKVILLE, Md., April 13, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today presented the preclinical results of ...
Ascentage Pharma Appoints Renowned Oncologist Dr. David Sidransky, MD, to its Board as an Independent Non-Executive Director
SUZHOU, China and ROCKVILLE, Md., April 2, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced its appointment of Dr.David ...
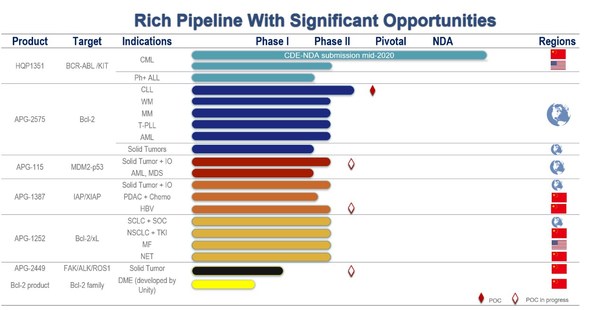
Ascentage Pharma Announces 2020 Annual Results and Reports Launch Readiness for Its Core Drug Candidate
SUZHOU, China and ROCKVILLE, Md., March 31, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today released its annual results for full ...
Ascentage Pharma Announces Publication of Preclinical Data in Nature Immunology Showing Enhanced T-Cell-Mediated Antitumor Immunity Induced by Its MDM2-p53 Inhibitor APG-115
SUZHOU, China and ROCKVILLE, Md., March 26, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced the peer-reviewed publicati...
Ascentage Pharma's First Third-Generation BCR-ABL Inhibitor in China Olverembatinib (HQP1351) Recommended for Breakthrough Therapy Designation
SUZHOU, China and ROCKVILLE, Md., March 24, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the Center for Drug Ev...
Ascentage Pharma to Present the Latest Results from Six Preclinical Studies at AACR Annual Meeting 2021
SUZHOU, China and ROCKVILLE, Md., March 11, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the latest preclinical...
Ascentage Pharma Released Preclinical Results of MDM2-p53 Inhibitor APG-115 in an Oral Presentation at WCLC 2020, Demonstrating Therapeutic Potential in STK11-Mutant Non-Small Cell Lung Cancer
SUZHOU, China and ROCKVILLE, Md., Feb. 2, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, announced that the company recently released ...
Ascentage Pharma Announces the Fifth Orphan Drug Designation Granted to Bcl-2 Inhibitor APG-2575 by the US FDA, and the Tenth Obtained by the Company
SUZHOU, China and ROCKVILLE, Md., Jan. 29, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the US Food and Drug A...
Ascentage Pharma Presents Updates on its Global Clinical Development at the J.P. Morgan 39th Annual Healthcare Conference
SUZHOU, China and ROCKVILLE, Md., Jan. 14, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, presented at the J.P. Morgan 39th Annual Hea...
Ascentage Pharma Announces its 9th Orphan Drug Designation from the US FDA in 2020, Setting a Record for Chinese Biopharmaceutical Companies
SUZHOU, China and ROCKVILLE, Md., Jan. 5, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the US Food and Drug Adm...
Ascentage Pharma's Licensee UNITY Biotechnology Announces Milestone Reached in Clinical Development of Treatments for Age-Related Disease, Leading to Milestone Payment
SUZHOU, China and ROCKVILLE, Md., Dec. 22, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that its licensee, UNITY Bi...
Ascentage Pharma Announces Updates on the Clinical Development of APG-2575, including an ORR of 70% in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia
SUZHOU, China and ROCKVILLE, MD, Dec. 10, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced updates on the clinical devel...
Ascentage Pharma Announces Positive Data from Pivotal Phase II Studies of HQP1351 (Olverembatinib) in Patients with TKI-Resistant Chronic Myeloid Leukemia (CML) at 2020 American Society of Hematology (ASH) Annual Meeting
SUZHOU, China, and ROCKVILLE, MD, Dec. 8, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today reported positive data from two pivotal...
Ascentage Pharma Appoints Mr. Gang Zhu as Chief Commercial Officer to Lead Commercial Initiatives
SUZHOU, China and ROCKVILLE, Md., Dec. 4, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced the appointment of Mr. Gang Z...
Ascentage Pharma Announces Clearances in China and the US for the Phase IIa Study of APG-115 Single Agent or in Combination with APG-2575 for the Treatment of Relapsed or Refractory T-cell Prolymphocytic Leukemia
SUZHOU, China and ROCKVILLE, Md., Dec. 2, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the Center for Drug Eval...
Ascentage Pharma Enters into an Agreement with University of Michigan to obtain an exclusive license for a MDM2 Degrader using PROTAC Technology
SUZHOU, China and ROCKVILLE, Md., Nov. 30, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced it has entered into an ag...
Ascentage Pharma Announces First Patient Dosed in Europe in the Phase Ib/II Study of the Bcl-2 inhibitor APG-2575 in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
SUZHOU, China and ROCKVILLE, Md., Nov. 25, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that a Phase Ib/II clinical ...
Ascentage Pharma Receives Approvals for Two Phase Ib/II Clinical Studies of the Bcl-2 Inhibitor APG-2575 for the Treatment of Waldenström Macroglobulinemia and Multiple Myeloma in China
SUZHOU, China and ROCKVILLE, Md., Nov. 23, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the Center for Drug Eva...
Ascentage Pharma to Release Updated Clinical Results of HQP1351 (Olverembatinib) in Drug-Resistant Chronic Myeloid Leukemia in an Oral Presentation at the American Society of Hematology Annual Meeting
SUZHOU, China and ROCKVILLE, Md., Nov. 9, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the results from two piv...
Ascentage Pharma Announces Approval for the Phase Ib/II Clinical Study of MDM2-p53 Inhibitor APG-115 in Combination with Immunotherapy for the Treatment of Patients with Advanced Liposarcoma or other Advanced Solid Tumors in China
SUZHOU, China and ROCKVILLE, Md., Oct. 27, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the Center for Drug Eva...