Kintor Pharmaceutical Receives Emergency Use Authorization for Proxalutamide for the Treatment of COVID-19 in Paraguay
SUZHOU, China, July 16, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced thatParaguay's Ministry of Public Health and Social Welfare (MSPBS) has granted an emerg...
Kintor Pharmaceutical Collaborates with Fosun Pharma Development to Commercialise Proxalutamide for Treatment of COVID-19 in India and Africa
SUZHOU, China, July 14, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939) today announced that it has entered into a licensing agreement with Shanghai Fosun Pharmaceutical Development Ltd. ("Fosun Pharma Development"), on the commercialisation of proxalutamide for the treatment of CO...
Kintor Pharmaceutical Receives IND Clearance by the U.S. FDA for GT20029 to Treat Androgenetic Alopecia and Acne
SUZHOU, China, July 13, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small-molecule and biological therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has cleared an Investigational New Drug...
Kintor Pharmaceutical Announced FDA Has Greenlighted Pyrilutamide's Phase II Clinical Trial for Androgenetic Alopecia in the US
SUZHOU, China, July 11, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has greenlighted pyrilutamide's phase...
Kintor Announced (1) FDA Has Greenlighted Proxalutamide's Phase III Study for Hospitalized Male and Female COVID-19 Patients to Be Conducted; and (2) Inclusion of Female Outpatients in Proxalutamide's Phase III Study for Mild to Moderate COVID-19
SUZHOU, China, May 19, 2021 /PRNewswire/ -- On May 18, Kintor Pharmaceutical Limited (HKEX.9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, announced that the U.S. Food and Drug Administration (FDA) has greenlighted proxalutamide's ph...
Kintor Pharmaceuticals Announced Successful Dosing of the First Batch of Patients for Acne Vulgaris Phase I/II Clinical Trial of Pyrilutamide
SUZHOU, China, April 16, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939), a clinical-stage biotechnology company developing small molecule and biological therapeutics, announced today the clinical trial of Pyrilutamide as a treatment for the acne vulgaris has completed the first b...
Kintor Pharmaceutical Announces AR-PROTAC (GT20029) Approved for Acne and Androgenic Alopecia Clinical Trials in China
SUZHOU, China, April 15, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (stock code 9939.HK, "Kintor Pharmaceutical" or the "Company"), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that it has received approval from t...
Kintor Pharmaceutical and Visum Announced Strategic Partnership to Expand Proxalutamide Manufacturing
SUZHOU, China, April 14, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (stock code 9939.HK, "Kintor Pharmaceutical" or the "Company"), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, recently announced a strategic partnership with Hain...
Kintor Pharmaceutical Limited Announces 2020 Business Progress and Annual Results
SUZHOU, China, March 26, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (stock code 9939.HK, "Kintor Pharmaceutical" or the "Company"), a clinical-stage biotechnology company developing small molecule and biological therapeutics, recently announced its business highlights and financial result...
Kintor Pharmaceutical Announces Results from Investigator-Initiated Brazil Trial Demonstrating 92% Reduction in Mortality in Hospitalized COVID-19 Patients
SUZHOU, China, March 11, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939), a clinical-stage biotechnology company developing small molecule and biological therapeutics, announced today top-line results from its investigator-initiated Brazil trial evaluating the efficacy of Proxalut...
Proxalutamide Phase III Clinical Trial for the Treatment of COVID-19 Patients Approved by FDA
SUZHOU, China, March 5, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to announce that the Investigational New Drug (IND) application of the phase III clinical trial of Proxalutamide's treatment of male COVID-19 outpatients has been approved by the United States Food ...
Completion of Patients' Recruitment for Proxalutamide's COVID-19 Hospitalised Study
SUZHOU, China, Feb.22, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939) is pleased to announce that the clinical trial of Proxalutamide for the treatment of hospitalised COVID-19 patients inBrazil completed the recruitment of 588 patients. The data of the clinical trial is expected ...
Kintor's GT20029 IND for PROTAC AR Degrader Was Accepted by NMPA
SUZHOU, China, Feb. 2, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX:9939) is pleased to announce that the investigational new drug ("IND") application of GT20029 for androgenetic alopecia and acne vulgaris indications has been accepted by the National Medical Products Administration (...
Clinical Trial of Proxalutamide's Trial of Hospitalized Covid-19 Patients Was Approved in Brazil
SUZHOU, China, Jan. 28, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to announce that the clinical trial (the "Trial") of Proxalutamide's treatment of hospitalized COVID-19 patients was approved by the Institutional Review Board ("IRB") ofBrazil. The trial was accept...
Kintor's Proxalutamide (GT0918) COVID-19 Clinical Trial Shows Positive Preliminary Results in Treatment of Female Patients
SUZHOU, China, Jan. 10, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the clinical trial of Proxalutamide for the treatment of COVID-19 patients. The investigator initiated trial conducted by Dr.Andy Goren and Dr. Flávio Adsuara C...
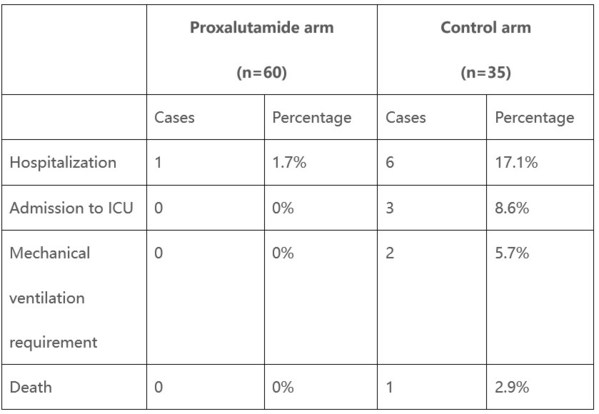
Kintor Proxalutamide's COVID-19 Clinical Trial Shows Significant Reduction in Hospitalization and Ventilation Rates
SUZHOU, China, Dec. 12, 2020 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the Proxalutamide's clinical trial for the treatment of COVID-19. Proxalutamide is a new androgen receptor (AR) antagonist developed in by Kintor Pharmaceutica...
Kintor Pharmaceutical Announces Preliminary Results from the Proxalutamide's Clinical Trial
SUZHOU, China, Dec. 11, 2020 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the Proxalutamide's clinical trial for COVID-19. Proxalutamide is a new androgen receptor (AR) antagonist developed in by Kintor Pharmaceutical and is currentl...
Stellar Results from Kintor's GT90001 and Opdivo Combo Therapy in the Second-line Treatment of Advanced Liver Cancer: ORR Reached up to 40%
SHANGHAI, Dec. 10, 2020 /PRNewswire/ -- On December 9, 2020, Kintor Pharmaceutical Limited (HKEx: 9939) is pleased to announce that the Group has collected positive data in phase II clinical trials of combination therapy of ALK-1 (GT90001) antibody and PD-1 (Nivolumab or Opdivo) antibody for the ...
Kintor Pharmaceutical's COVID-19 Clinical Trials for Proxalutamide to expand Patient Enrolment
SUZHOU, China, Oct. 29, 2020 /PRNewswire/ -- Kintor Pharmaceutical Limited (Kintor Pharmaceutical, stock code 9939.HK) is delighted to announced that the clinical trial (ClinicalTrials.gov identifier: NCT04446429, registered by Dr. Andy Goren and Applied Biology) of its anti-androgen treatment Pro...