Seegene Earns Improved ESG Rating from the Korea Institute of Corporate Governance and Sustainability
Company attributes B+ rating to progress related to its Human Rights Management Declaration, eco-friendly shipping box and the establishment of an internal ESG Committee SEOUL, South Korea, Nov. 10, 2023 /PRNewswire/ -- Seegene Inc. (KQ096530), a leading South Korean company providing a total so...
Seegene Reinforces Commitment to Strong ESG Management with 100% Recyclable, Styrofoam-Free Shipping Boxes
* 100% recyclable package made with eco-friendly and FSC-certified materials to proactively meet evolving global environmental regulations * Patent design provides a 30% increase in load capacity and an up to 52% reduction in transportation costs * This initiative is part of Seegene's effort...
Seegene Unveils 'Open Innovation Program Powered by Seegene' in Partnership with Springer Nature
* The program is a part of the Seegene OneSystemTM business aimed at creating a 'world free from all diseases' * Collaborating with clinical researchers worldwide, Seegene will share its technologies and enable researchers to develop syndromic qPCR assays SEOUL, South Korea, Sept. 4, 2023 /PR...
Seegene unveils solutions to popularize molecular diagnostics at 2023 AACC
* Introduced syndromic quantitative PCR assays and a fully automated molecular diagnostic testing system, STARlet AIOS™ * Conducted symposium sessions on the usefulness of PCR testing in diagnosing gastrointestinal diseases * Participated in a presentation event for major Korean in-vitro dia...
Springer Nature to Join Seegene's OneSystem™ Business as Strategic Partner
* Seegene announces strategic partnership with Springer Nature to expand OneSystem™ Business * Seegene and Springer Nature to jointly launch global assay development project * Springer Nature expresses active support for Seegene's vision to promote participation across global scientific com...
Seegene and Werfen Partner to Collaborate on OneSystem™ Business to Develop Syndromic qPCR Assays
SEOUL, South Korea, June 14, 2023 /PRNewswire/ -- Seegene Inc. (KQ096530), a leading South Korean company which provides a total solution for PCR molecular diagnostics, announced an agreement with Werfen, a worldwide leader in Specialized Diagnostics, to advance discussions on the expansion of it...
Seegene Obtains IVDR Certification for 30 Diagnostic Assays
* Seegene certifies diagnostic assays for digestive, respiratory, women's diseases, and human papillomavirus to meet IVDR requirements * New regulation sets improved standards to ensure higher level of quality, safety, and reliability SEOUL, South Korea, June 1, 2023 /PRNewswire/ -- Seegene I...
Seegene declares to share Syndromic PCR technologies to prevent future pandemics
* Localization of Seegene Syndromic PCR technologies with global partners for versatile and proactive response to local emerging healthcare demands. * Formation of a collaborative global network to aggressively develop innovative syndromic PCR assays, challenging boundaries of PCR application....
Seegene reports H1 2022 financial results
* H1 revenue fell 11% YoY to KRW 579.9 billion, operating profit down 37% to KRW 212.7 billion * Decline due to drop in Q2 sales amid reduced PCR testing volume * Sales of non-COVID products and PCR instruments increased in H1; Company launches STARlet-AIOS to strengthen sales foundation *...
Seegene unveils strategies for global expansion at 2022 AACC
Company has taken part in the Annual Scientific Meeting & Clinical Lab Expo since 2007 This year's event showcased its In-life PCR campaign and STARlet-AIOS Seegene also displayed Novaplex™ assays, including a test for detecting the monkeypox virus SEOUL, South Korea, July 29, 2022 /PRNewswire/...
Seegene launches world's first In-life PCR campaign in Vietnam
* Test center LabHouse and ride-hailing service Grab to start PCR testing for Grab drivers in August * Seegene to provide Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay to simultaneously detect COVID-19, flu, cold * Testing to begin with drivers in Ho Chi Minh, Hanoi and will later be expanded to ...
Seegene to pave way for PCR testing at local clinics with EU-approved multiplex test and fully automated PCR solution
SEOUL, South Korea, June 29, 2022 /PRNewswire/ -- Seegene Inc. (KQ096530), South Korea's leading molecular diagnostics (MDx) company, has obtained EU approval for its Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay that is compatible with the company's fully automated 'AIOS' (All-in-One System). This is ...
Seegene develops PCR test to detect monkeypox virus
* Assay targets monkeypox virus and can deliver results in 90 minutes * Company swiftly rolls out product using its automated assay development system * "Seegene will strive to make accurate tests for emerging viruses to help prevent future pandemics" SEOUL, South Korea, June 28, 2022 /PRN...
Seegene unveils '$12 PCR testing' initiative to help end COVID-19 pandemic
Includes asymptomatic testing at community-based facilities to prevent widespread transmissions Regular testing for COVID-19, flu, RSV crucial for staying safe as anti-virus restrictions are eased PCR tests will be priced affordably, made possible through Seegene's 20 years of expertise "Full ...
Seegene reports first quarter 2022 financial results
* Q1 revenue up 10% QoQ to reach record quarterly high of KRW 451.5 billion * Foundation for sales expansion strengthened through '3 Ct' technology commercialization, Allplex™ RV Master Assay approval, increased PCR instrument sales * "Seegene to secure future growth by targeting syndromic t...
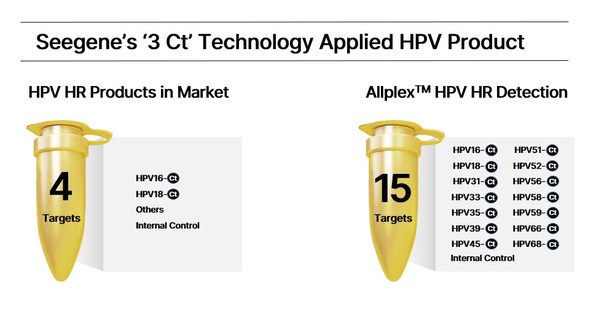
Seegene unveils world's first commercialized '3 Ct' PCR assay
* Provides Ct value of three targets in one channel; '3 Ct' PCR assay to launch in H1 * "Dream MDx technology" developed based on Seegene's 20-year expertise; combines 19 different patented technologies, including DPO™, TOCE™, MuDT™ * '3 Ct' to lay foundation for automated syndromic testing ...
Seegene's Allplex™ RV Master Assay receives Australian TGA approval, European CE-IVD mark
* Test detects 21 targets for 19 respiratory viruses in single tube, including COVID-19 and flu * Assay result of Seegene's decades-long know-how, applies DPO∙TOCE∙MuDT technologies * "Optimal solution for safe return to normalcy as distancing restrictions eased" SEOUL, South Korea, April 6...
Seegene appoints Dr. Glen Hansen as Head of Medical Affairs of U.S. subsidiary
SEOUL, South Korea, March 17, 2022 /PRNewswire/ -- Seegene Inc.
Seegene appoints Richard Creager as CEO of U.S. subsidiary to bolster U.S. business
SEOUL, South Korea, March 2, 2022 /PRNewswire/ -- Seegene Inc.
Seegene unveils new assay optimized for on-site testing in 'living with Covid-19' era
SEOUL, South Korea, Feb. 24, 2022 /PRNewswire/ -- Seegene Inc. (KQ 096530), South Korea's leading molecular diagnostics company, today, announced the CE-IVD marking of its Allplex™ SARS-CoV-2 fast MDx Assay. This new assay is optimized for the 'living with COVID-19' era, where mass, swift and ...