ZEISS expands DORC portfolio and further strengthens digital footprint with improved workflow efficiency and digital visualization
Showcasing at AAO 2025: * NEW 510k CLEARANCE SUPPORTS MORE INFORMED DECISION MAKING & PATHOLOGY DETECTION: Showcasing optical coherence tomography angiography (OCT-A) with ZEISS CIRRUS AngioPlex® with noninvasive imaging of the retinal and choroidal vasculature; now with510k clearance and CE a...
ZEISS showcases expansion of ophthalmic care options creating industry-leading workflow solutions; marks new refractive and cataract milestones
ZEISS Medical Technology will showcase new ophthalmic innovations and market milestones at ESCRS fromSept. 12 - 15 in Copenhagen, Denmark: * EFFICIENT, CONFIDENT DECISION MAKING: introducing AI-powered CIRRUS® PathFinder™ clinical support tool now CE mark approved; presenting a fully integrate...
ZEISS showcases comprehensive workflow for full spectrum of retina care at EURETINA
At EURETINA 2025, ZEISS Medical Technology combines its diagnostic, surgical and digital technologies to support healthcare professionals in advancing retinal care: * IMPROVE PATHOLOGY DETECTION: Now CE mark approved, CIRRUS® PathFinderTM AI decision support tool streamlines OCT data review; I...
ZEISS announces CE mark for CIRRUS PathFinder AI tool with automated OCT assessment
Fully integrated AI decision support tool flags B scans that may require further review, supporting more confident decision making and more efficient patient care. JENA, Germany, Aug. 21, 2025 /PRNewswire/ -- ZEISS Medical Technology today announced CE mark approval forCIRRUS® PathFinder™, an in...
ZEISS continues to drive digital era forward in ophthalmology; celebrates 2 million digitally-planned cataract cases in the U.S. alone
Showcasing at ASCRS 2025: * More than 2 million cases planned with ZEISS VERACITY Surgery Planner: Growing use of one of the leading digital planning solutions for cataract surgery in the U.S. is a testament to the increased adoption of the ZEISS Cataract Workflow digital solutions by surgeons...
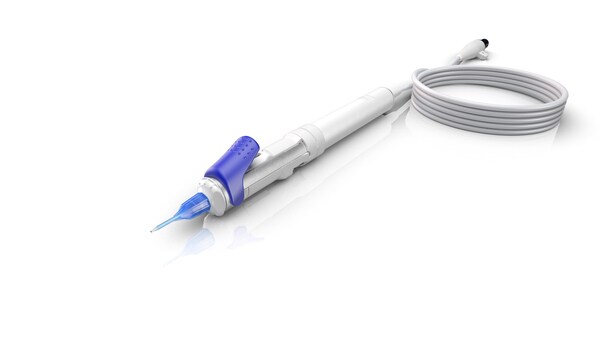
ILM-Blue from DORC Receives NMPA Approval in China; Expanding Global Reach
JENA, Germany, March 27, 2025 /PRNewswire/ -- ZEISS Medical Technology announced today that the ILM staining dye ILM-Blue® from DORC (Dutch Ophthalmic Research Center (International) B.V.) has received National Medical Products Administration (NMPA) approval inChina, marking a significant milesto...
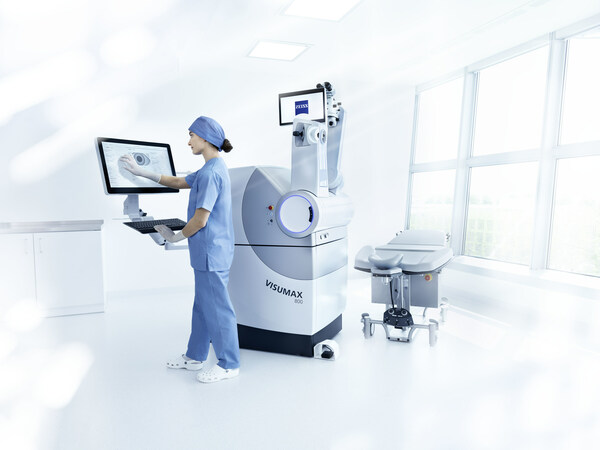
ZEISS VISUMAX 800 with SMILE pro software receives approval in China
Updated ZEISS femtosecond laser provides refractive surgeons in China with faster treatment, greater flexibility, and significant workflow JENA, Germany and SHANGHAI, Feb. 27, 2025 /PRNewswire/ -- ZEISS Medical Technology announced today that the National Medical Products Administration (NMPA) ...
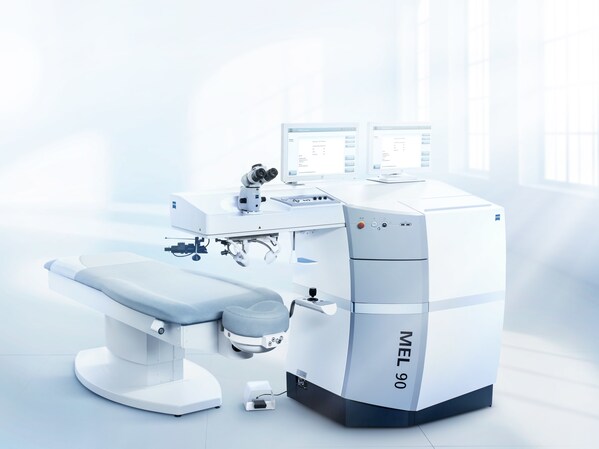
ZEISS MEL 90 excimer laser receives U.S. FDA approval; completes Corneal Refractive Workflow
The excimer laser complements the ZEISS VISUMAX 800 with ZEISS SMILE pro, extending ZEISS' LVC market leadership with treatment for myopia, hyperopia, and mixed astigmatism. DUBLIN, Calif. and JENA, Germany, Jan. 13, 2025 /PRNewswire/ -- ZEISS Medical Technology announced today that theMEL® 90 r...
ZEISS expands ophthalmic offerings to improve patient care with new digital AI tools and revolutionary surgical solutions
Showcasing at AAO 2024: * ZEISS VisioGen offers a cutting-edge, AI-driven solution designed to enhance refractive patient communication and streamline clinic operations. * ZEISS Milestone: Celebrating more than 10 million eyes treated with lenticule extraction solutions utilizing ZEISS SMIL...
ZEISS reinvents lens extraction with the first hand-held ultrasound-free lens removal device, available in the U.S.
The ZEISS MICOR 700 uses the ZEISS NULEX (non-ultrasonic lens extraction) procedure to help surgeons broaden their intraocular working space, minimizing risk to surrounding eye structures and increasing operating room efficiency. DUBLIN, Calif. and JENA, Germany, Sept. 26, 2024 /PRNewswire/ -- ZE...
ZEISS Redefines Disease Management and Treatment within the ZEISS Retina Workflow
Showcasing at EURETINA 2024: ZEISS will highlight new surgical innovations and artificial intelligence (AI) tools for retinal patient care, helping doctors diagnose and treat patients efficiently and effectively: * THE FUTURE OF VITREORETINAL SURGERY: Demonstrating the ZEISS ARTEVO 850 3D dig...
ZEISS innovation paves way to more personalized patient care with cutting-edge AI technology and industry-leading surgical solutions
Showcasing at ESCRS from Sept. 6 to 10, in Barcelona, Spain: REFRACTIVE INDUSTRY MILESTONES: ZEISS extends its global refractive industry momentum with more than 10 million eyes treated with ZEISS SMILE and ZEISS SMILE pro; ZEISS announces first treatment in the CE market for hyperopia with len...
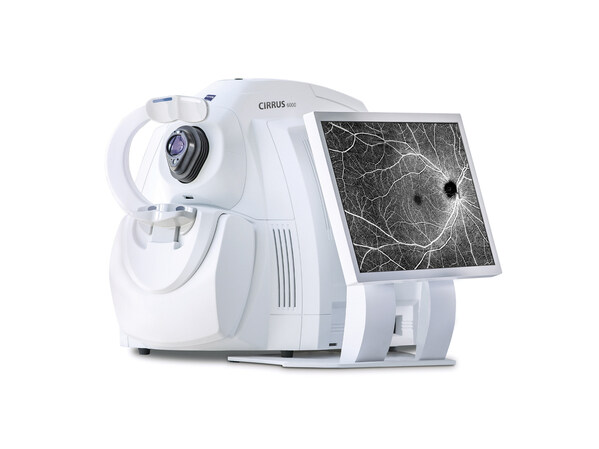
ZEISS announces OCT technology enhancements to better support growing era of data-driven patient care
ZEISS Medical Ecosystem fortifies the CIRRUS 6000 with the U.S. market's largest OCT reference database and advanced cybersecurity features. DUBLIN, Calif., May 30, 2024 /PRNewswire/ -- ZEISS Medical Technology announced today that the CIRRUS® 6000 from ZEISS now enables a highly efficient and d...
Carl Zeiss Meditec AG Completes Acquisition of Dutch Ophthalmic Research Center (D.O.R.C.); Companies Unite to Shape Ophthalmology Market
ZEISS secures regulatory approvals to acquire D.O.R.C.; companies now shift focus to integration implementation, fueling world-class innovation, and driving market expansion strategy for ophthalmic medical devices and surgery. JENA, Germany and DUBLIN, Calif., April 4, 2024 /PRNewswire/ -- Carl...
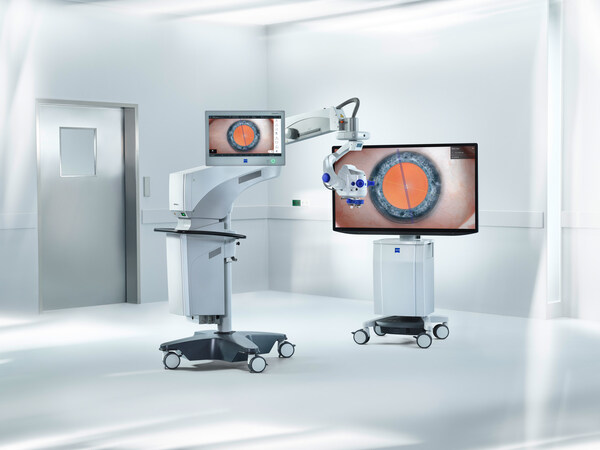
ZEISS Sets Stage for Future of Ophthalmic Surgery and 3D Visualization at ASCRS 2024
* Product News: ZEISS announces enhanced visualization capabilities with the new ZEISS ARTEVO 850 3D heads-up ophthalmic microscope. * Spatial Computing Innovation: ZEISS will demonstrate a digital application for experiencing 3D videos and more on the Apple Vision Pro. * Workflow Innovati...
U.S. FDA Approves the VISUMAX 800 with SMILE pro software from ZEISS
The updated ZEISS femtosecond laser provides U.S. refractive surgeons with faster treatment, greater flexibility, and significant workflow enhancements. DUBLIN, Calif. and JENA, Germany, Jan. 11, 2024 /PRNewswire/ -- Carl Zeiss Meditec AG -- ZEISS Medical Technology announced today that the U.S. ...
Carl Zeiss Meditec AG announces agreement to acquire Dutch Ophthalmic Research Center (D.O.R.C.)
Acquisition will extend the company's leadership in ophthalmic medical devices market and expands its position in the vitreo-retinal surgery segment JENA, Germany, Dec. 16, 2023 /PRNewswire/ -- Carl Zeiss Meditec AG announced today that it has entered into an agreement to acquire 100% of the shar...
ZEISS establishes the patented SMILE technology with 1.5 million laser vision corrections worldwide
ZEISS announces two new milestones in refractive technology, with 1.5 million SMILE treatments and the production of its 1000th VisuMax femtosecond laser JENA, Germany, Sept. 21, 2018 /PRNewswire/ -- Today, the Medical Technology business group of ZEISS announced two milestones in refractive tech...
ZEISS Presents Integrated Data-Driven Digital Solutions at ESCRS, Advancing Eye Care for Patients Every Step of the Way
During the combined exhibition of the European Society of Cataract & Refractive Surgeons (ESCRS) and the European Society of Retina Specialists (EURETINA), ZEISS presents its cross-platform ophthalmic digital portfolio which aids doctors in disease management, allowing them to help their patients...