Once-Monthly GLP-1 RA | Gan & Lee Pharmaceuticals Initiates Phase 3 Clinical Study (GRADUAL-3) of the First Chinese Once-Monthly GLP-1 RA for Weight Management
BEIJING, Nov. 27, 2025 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, stock code: 603087.SH) announced the initiation of GRADUAL-3, its third large-scale phase 3 clinical study of the glucagon-like peptide-1 receptor agonist (GLP-1 RA) bofanglutide (research code: GZR18) injection in adult...
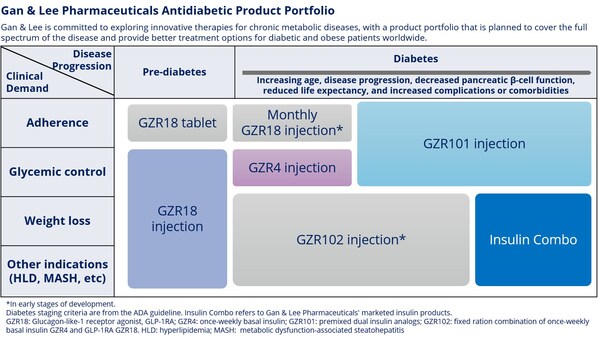
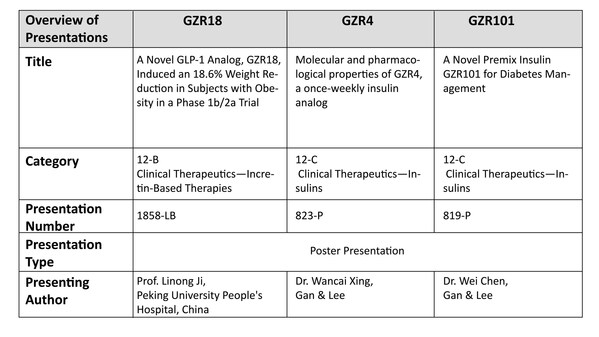
Gan & Lee Pharmaceuticals Presented Multiple Results in Novel Diabetes Therapies at the American Diabetes Association's 85th Scientific Sessions
* In a Phase 2a clinical trial, the GLP-1 RA bofanglutide injection demonstrated a favorable safety and tolerability profile after 23 weeks of once weekly treatment in patients with type 2 diabetes mellitus (T2DM), with significant HbA1c reductions alongside comprehensive benefits for body weig...
Gan & Lee Pharmaceuticals Announced First Participant Dosed in the US Phase 2 Clinical Study of Bofanglutide (GZR18) Injection for Overweight or Obesity
BEIJING and BRIDGEWATER, N.J., March 10, 2025 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced that the first participant has been successfully dosed in a Phase 2 clinical trial of bofanglutide (research code: GZR18) injection, a bi-weekly glucagon-...
Gan & Lee Pharmaceuticals Announces U.S. FDA Clearance of the IND application for the innovative Bi-weekly GLP-1RA GZR18 Injection, Bofanglutide, with chronic weight management Indication (A Phase 2 head-to-head with Tirzepatide clinical trial)
BEIJING and BRIDGEWATER, N.J., Dec. 18, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087.SH), is pleased to announce that the Food and Drug Administration (" FDA ") has cleared the Investigational New Drug (IND) application for GZR18 Injection to conduct ...
Gan & Lee Pharmaceuticals Announced Positive Phase 1 Results for Its Oral GLP-1 Receptor Agonist GZR18 Tablet in Healthy Participants, Demonstrating 4.16% Weight Reduction in Two Weeks
* After two weeks of treatment, GZR18 tablets resulted in an average weight reduction of up to 4.16%, with continued weight loss observed even after discontinuation. * The pharmacokinetic profile supports a once-daily oral dosing regimen for GZR18 tablets. * The safety and tolerability of G...
ObesityWeek®️ 2024 | Gan & Lee Pharmaceuticals Orally Presents Phase 2b Clinical Data for GZR18 Injection in Chinese Overweight and Obese Adults
* Obese or overweight participants receiving bi-weekly doses of 12 mg, 18 mg, 24 mg, 48 mg, and once-weekly dose of 24 mg GZR18 for 30 weeks achieved mean percent changes in body weight from baseline of -11.15%, -13.22%, -14.25%, -17.29%, and -17.78%, respectively, with the placebo group at -0....
Gan & Lee Pharmaceuticals' Three Innovative Drugs: GZR18 Injection, GZR4 Injection, and GZR101 Injection Achieve Primary Endpoints in Phase 2 Clinical Studies
* After 24 weeks of treatment in patients with Type 2 diabetes, the bi-weekly GLP-1 receptor agonist GZR18 injection showed superior efficacy in lowering HbA1c and body weight compared to semaglutide (Ozempic®). * After 16 weeks of treatment in patients with Type 2 diabetes, the once-weekly i...
Gan & Lee Pharmaceuticals Presented Two Positive Clinical Results of Once-weekly Insulin GZR4 at the 60th Annual Meeting of the European Association for the Study of Diabetes (EASD 2024)
* In Phase Ia clinical study, GZR4 demonstrated favorable safety and tolerability profiles in healthy subjects, maintaining a stable glucose-lowering effect for up to one week with a single administration. * In Phase Ib clinical study, patients with Type 2 diabetes mellitus (T2DM) receiving s...
Gan & Lee Pharmaceuticals' Bi-weekly (twice a month) GLP-1 Receptor Agonist GZR18 Injection Achieved 17.29% Weight Loss at 30 Weeks in a Phase IIb Clinical Trial
* Obese and overweight participants receiving bi-weekly doses of 12 mg, 18 mg, 24 mg, 48 mg, and once-weekly dose of 24 mg GZR18 for 30 weeks achieved mean percent changes in body weight from baseline of -11.15%, -13.22%, -14.25%, -17.29%, and -17.78%, respectively, with the placebo group at -0...
Gan & Lee Pharmaceuticals Announces Significant Progress on New Diabetes and Obesity Treatments at the American Diabetes Association's 84th Scientific Sessions
BEIJING and BRIDGEWATER, N.J., June 23, 2024 /PRNewswire/ -- Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced the results of the Phase1b/2a clinical study of the Company's independently developed glucagon-like peptide-1 (GLP-1) receptor agonist, GZR18 Injection, in...
Gan & Lee Pharmaceuticals to Present Groundbreaking Data on Three Innovative Products at the American Diabetes Association's 84th Scientific Sessions
* A phase 1b/2a trial evaluated once- and bi-weekly GZR18, a novel GLP-1 analog, in Chinese subjects with obesity/overweight * Pre-clinical studies evaluated the molecular and pharmacological properties of GZR4, an investigational once-weekly insulin analog * Pre-clinical studies evaluated ...
Qiming's Portfolio Company InventisBio Lists on STAR Market
SHANGHAI, July 25, 2022 /PRNewswire/ -- Qiming Venture Partners' portfolio company InventisBio (SHSE: 688382), a leading company in innovative drug development, today listed on the Science and Technology Innovation Board (the "STAR Market") of the Shanghai Stock Exchange. The issue price isCNY 18...
Qiming's Portfolio Company InventisBio Lists on STAR Market
SHANGHAI, July 24, 2022 /PRNewswire/ -- Qiming Venture Partners' portfolio company InventisBio (SHSE: 688382), a leading company in innovative drug development, today listed on the Science and Technology Innovation Board (the "STAR Market") of the Shanghai Stock Exchange.The issue price is CNY 18...
Qiming Venture Partners Announces Closing of Funds Totaling US$3.2 Billion
SHANGHAI, July 11, 2022 /PRNewswire/ -- Qiming Venture Partners announced today the closing of its latest funds totalingUS$3.2 billion, including USD Fund VIII atUS$2.5 billion and the first closing of RMB Fund VII at RMB 4.7 billion (or US$ 700 million). As in prior funds, Fund VIII was oversub...
Qiming's Nisa Leung, Duane Kuang Named on 2022 Forbes Midas List
SHANGHAI, April 13, 2022 /PRNewswire/ – Forbes released the 2022 Midas List on April 12, a ranking of the world's top 100 venture capitalists. Nisa Leung, Managing Partner of Qiming Venture Partners, andDuane Kuang, Founding Managing Partner of Qiming Venture Partners, ranked on the list. Since ...
Gan & Lee Pharmaceuticals Begins First-In-Human Trial in U.S. for Investigational Drug, GZR18
BEIJING and BRIDGEWATER, N.J., March 11, 2022 /PRNewswire/ -- Gan & Lee Pharmaceuticals Co., Ltd. (hereinafter referred to as Gan & Lee, stock code: 603087.SH), is pleased to announce the first subject has been dosed in our Phase I, double-blinded, randomized, placebo-controlled, sequential, sing...
Qiming Venture Partners Announces Annual Promotions
SHANGHAI, Jan. 7, 2022 /PRNewswire/ -- Qiming Venture Partners, a leading venture capital firm inChina, is pleased to announce the promotions of Bonnie Wang and Dr. Kan Chen to Partner, and Xiaofei Zhou to Vice President. In addition, there are eight promotions from the finance, legal and adminis...
Gan & Lee Pharmaceuticals Announces U.S. FDA Clearance of Investigational New Drug (IND) Application for a Novel Glucagon-Like Peptide-1 Analogue, GZR18
BEIJING and BRIDGEWATER, N.J., Dec. 7, 2021 /PRNewswire/ -- Gan & Lee Pharmaceuticals Co., Ltd. (hereinafter referred to as Gan & Lee, stock code: 603087.SH), is pleased to announce that the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for the...
Gan & Lee Pharmaceuticals Concludes Phase 3 Studies of Gan & Lee Insulin Glargine (GL-GLA)
BEIJING and BRIDGEWATER, N.J., Oct. 14, 2021 /PRNewswire/ -- Gan & Lee Pharmaceuticals Co., Ltd. (hereinafter referred to as Gan & Lee) (Shanghai: 603087.SH), is pleased to announce the completion of two randomized, multicenter, phase 3 studies that compare proposed biosimilar Gan & Lee insulin ...
Gan & Lee Pharmaceuticals to present data at the 57th Annual European Association for the Study of Diabetes Meeting
BEIJING and BRIDGEWATER, N. J., Sept. 9, 2021 /PRNewswire/ -- Gan & Lee Pharmaceuticals Co., Ltd. (hereinafter referred to as Gan & Lee, stock code: 603087.SH), is excited to present positive data from five abstracts which will be featured as short oral discussions at the 57th Annual European Ass...