Mabwell's Novel Nectin-4 Targeting ADC 9MW2821 Approved for 2 Clinical Trials by CDE of NMPA
SHANGHAI, Nov. 14, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its novel Nectin-4 targeting ADC (R&D code: 9MW2821) has received IND approval for two clinical studies. The first will explore its use in combination wi...
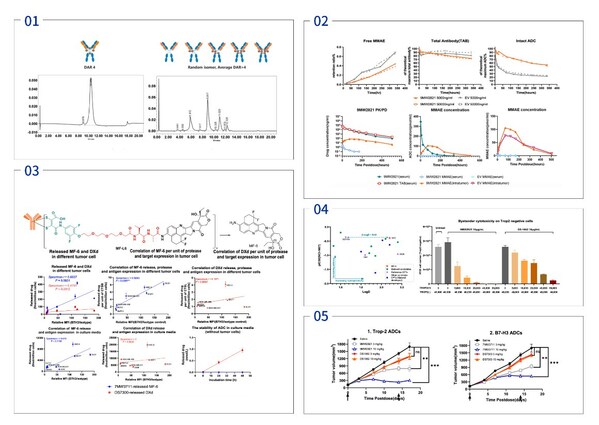
Mabwell Reveals Encouraging Pre-clinical Data of 7MW4811 at the 15th World ADC Conference
SHANGHAI, Nov. 8, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, showcased the "Preclinical Development of 7MW4811, an Mtoxin™ (MF6)-based Antibody-drug Conjugate for the Treatment of Solid Tumors" as a poster presentation at the 15 t...
Mabwell Announces CDE Approval to Initiate Phase III Clinical Trial of 9MW2821 for Urothelial Carcinoma in Combination with PD-1 Inhibitor
SHANGHAI, Aug. 26, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its submission to the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) for "a randomized, controlled, open-label, ...
Mabwell Announces the CDE Approval of Novel Nectin-4 Targeting ADC to Initiate Phase III Clinical Trial for the Treatment of Cervical Cancer
SHANGHAI, Aug. 23, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its submission to the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) for the "A Randomized, Open-label, Phase II...
Mabwell's Novel Nectin-4 Targeting ADC 9MW2821 Granted Breakthrough Therapy Designation by China's NMPA
SHANGHAI, Aug. 12, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821) has been granted Breakthrough Therapy Designation (BTD) bythe Center for Drug Evaluation (CDE) of China's...
FDA Grants Orphan Drug Designation to 7MW3711
SHANGHAI, July 16, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that its self-developed novel B7-H3-targeting ADC (R&D code: 7MW3711) has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Adminis...
Mabwell Receives NMPA Approval for Clinical Trial of Novel Nectin-4 Targeting ADC in TNBC
SHANGHAI, July 15, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821) has been approved by the NMPA to enter Phase II clinical trial as monotherapy or in combination with a PD...
Mabwell Announces 9MW2821 Clinical Data and Latest Progress to be presented at 2024 ASCO Annual Meeting
SHANGHAI, May 24, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company, announced the data and latest progress of the Phase I/II clinical study of 9MW2821, a novel Nectin-4-targeting ADC for multiple advanced solid tumors, which will be reported as an oral pre...
Mabwell Announces Clinical Trial Progress of 9MW2821 in Triple-Negative Breast Cancer
SHANGHAI, May 13, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced the progress of the clinical study of its novel Nectin-4-targeting ADC (R&D code: 9MW2821) for triple-negative breast cancer. Based on 9MW2821's curren...
FDA Grants Orphan Drug Designation to 9MW2821
SHANGHAI, May 7, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that its self-developed novel Nectin-4-targeting ADC (R&D code: 9MW2821) has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Adminis...
2024 ASCO | Mabwell to Present Clinical Data of 9MW2821 in Multiple Advanced Solid Tumor
SHANGHAI, April 25, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with an entire industry chain, announced that the Phase I/II clinical study results of the novel Nectin-4-targeting ADC 9MW2821 for multiple advanced solid tumors will be presented in an ...
Mabwell Releases Preclinical Study Results of Multiple Innovative Drugs Released at the 2024 AACR Annual Meeting
SHANGHAI, April 16, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, presented the preclinical research findings of three potential novel drug candidates in poster format at the American Association for Cancer Research (AACR) An...
MAIWEIJIAN, First Approved Biosimilar of Denosumab (120mg) in China
SHANGHAI, April 8, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that Denosumab Injection (trade name: MAIWEIJIAN, R&D code: 9MW0321) developed by its wholly-owned subsidiary T-mab has officially obtained the market...
Mabwell Presented IDDC™ Platform Technology and ADC Drug Development Achievements at the 14th World ADC London
SHANGHAI, March 21, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, published "Mtoxin™ Payload Applied in IDDC™ ADC Platform Significant Increases Therapeutic Index and Overcome MultiDrug Resistance in Various Tumor" as poster ...
SGO 2024 | The First Published Clinical Data of Nectin-4-Targeting ADC Developed by Mabwell in Cervical Cancer Demonstrates Its Outstanding Therapeutic Potential
SHANGHAI, March 19, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative-driven biopharmaceutical company with entire industry chain, presented the clinical study data of the 9MW2821 for patients with cervical cancer as focused plenary oral presentation at the Society of Gynecologic Oncology ...
Mabwell to Present Pre-clinical Results at the 2024 American Association for Cancer Research (AACR) Annual Meeting
SHANGHAI, March 12, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present results of three preclinical studies as poster presentation at the AACR Annual Meeting to be held inSan Diego, USA, from April 5...
Mabwell to Present ADC Platform IDDC™ and the Latest Study Results of Multiple Novel ADCs at the 14th World ADC London
SHANGHAI, March 11, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present its next generation ADC platform IDDC™ and the latest study results of multiple novel ADCs (9MW2821, 7MW3711, 9MW2921) developed...
Mabwell to Present the Clinical Data of 9MW2821 in Cervical Cancer as Focused Plenary Oral Presentation at 2024 Society of Gynecologic Oncology Annual Meeting on Women's Cancer
SHANGHAI, March 8, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present the clinical study results on the efficacy and safety of the novel Nectin-4-targeting ADC 9MW2821 for patients with recurrent or ...
FDA Grants Fast Track Designation to 9MW2821
SHANGHAI, Feb. 27, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announces that its self-developed novel ADC drug targeting Nectin-4 (R&D Code: 9MW2821) has been granted Fast Track Designation (FTD) by the U.S. Food and Drug Administ...
Mabwell Publishes the Phase III Study Results on Its Denosumab Biosimilar (MW032) in the journal JAMA Oncology
SHANGHAI, Feb. 21, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, recently published the phase III study results of denosumab biosimilar (MW032) online in the international top journal of JAMA Oncology. This is the first recorded tria...