Biotechnology
Oncoclínicas Group records a net revenue of BRL 41 million for Q123
The company reported 171 million for the last twelve months, with a 60% growth in net revenue and its best EBITDA ever SAO PAULO, May 22, 2023 /PRNewswire/ -- Oncoclínicas Group (ONCO3) - the largest group dedicated to cancer treatment inLatin America - announced its results for the first quarte...
One BioMed Announce World's First Fully Integrated Platform for Automated High Molecular Weight DNA Extraction
Beyond Simple HMW DNA extraction at scale. SINGAPORE, May 22, 2023 /PRNewswire/ -- One BioMed, announced today the launch of the X8™ HMW DNA Cartridge Kits for the automated extraction of high molecular weight DNA for use in long-read sequencing and genome assembly. Inclusive of all the necessa...
Laekna announces first IND clearance by U.S. FDA for internally-discovered LAE102
SHANGHAI and WARREN, N.J. , May 21, 2023 /PRNewswire/ -- Laekna, a clinical-stage biotechnology company dedicated to bringing novel therapies to cancer and liver fibrosis patients worldwide, announced today that the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug ...
Fresh2 Group Ltd. Announces Nasdaq Ticker Symbol Change from 'ANPC' to 'FRES'
Ticker symbol change effective at the market open May 22, 2023 NEW YORK, May 19, 2023 /PRNewswire/ -- Fresh2 Group Ltd., formerly AnPac Bio-Medical Science Co., Ltd. (NASDAQ: ANPC), a company with operations in the United States and China focused on early cancer screening and detection and plans...
Positive Results from Interim Analysis of 'GEN-001' Plus avelumab (Bavencio®) Phase II Trial
* Positive primary endpoint result of objective response rate (ORR) from the planned interim analysis of Phase II trial in advanced gastric/gastroesophageal junction adenocarcinoma (GC/GEJ). * Top-line data of the final analysis from Phase II to be announced in the second half of next year. S...
HanAll Biopharma Announces Results from Phase III randomized, double-masked active treatment-controlled VELOS-3 Trial Evaluating Tanfanercept 0.25% for Treatment of Dry Eye Disease
* Tanfanercept did not demonstrate statistical significance in either of the primary outcome measures of improvement in central corneal staining score (CCSS) or in improvement in Eye Dryness Score (EDS) assessed at week 8 in subjects with dry eye disease (DED), compared to vehicle. * However,...
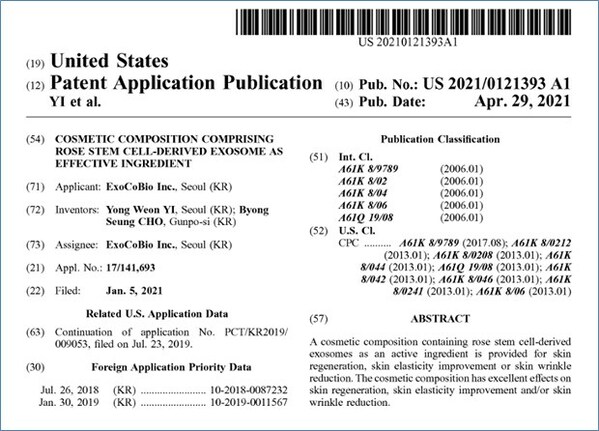
ExoCoBio obtained a US Patent for Rose Stem Cell-derived Exosome (RSCE™)
- The first plant stem cell-derived exosome patent in the US - The 5th patent allowance following 3 in Korea and 1 in Japan - An important milestone for the global plant stem cell-derived exosome industry SEOUL, South Korea, May 19, 2023 /PRNewswire/ -- ExoCoBio, one of the top three exosome...
Positive Results from Interim Analysis of 'GEN-001' Plus avelumab (Bavencio®) Phase II Trial
SEOUL, South Korea, May 19, 2023 /PRNewswire/ -- Genome & Company (CEO: Pae, Jisoo), a global leading microbiome therapeutics developer, announced that this phase II trial (NCT05419362) will continue without modifications to move on to the second stage, based on the positive result of the interim...
HitGen Announces Drug Discovery Research Agreement with ARase Therapeutics
CHENGDU, China, May 18, 2023 /PRNewswire/ -- Shanghai Stock Exchange listed company HitGen Inc. ("HitGen", SSE: 688222.SH) announced today that it has entered into a research agreement with ARase Therapeutics, Inc. ("ARase"), a biotechnology company developing first-in-class therapeutics targetin...
YS Biopharma to Participate in the Virtual Benchmark Healthcare House Call Conference on May 23, 2023
GAITHERSBURG, Md., May 18, 2023 /PRNewswire/ -- YS Biopharma Co., Ltd. (NASDAQ: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious diseas...
DISCOVERY LIFE SCIENCES JOINS AKOYA BIOSCIENCES' GLOBAL SERVICE PROVIDER NETWORK TO ACCELERATE IMMUNO-ONCOLOGY RESEARCH
HUNTSVILLE, Ala., May 18, 2023 /PRNewswire/ -- Discovery Life Sciences™ (Discovery), the Biospecimen and Biomarker Specialists™, announced today that it has officially qualified as one of Akoya Biosciences' global service providers. The qualification process evaluated Discovery's proficiency in ...
Top-quality medical grade TPUs enabled by smart factory: ICP DAS - BMP to exhibit at Medtec China 2023
HSINCHU, May 18, 2023 /PRNewswire/ -- Smart manufacturing & stringent process control enhance TPU quality. ICP DAS - BMP (Biomedical Polymers), aTaiwan-based supplier of medical-grade TPU (thermoplastic polyurethane), acts as a front-runner who deploys IIoT systems in a smart factory to maintain ...
Asieris Deepens Strategic Partnership with UroViu to Further Develop Integrated Diagnosis and Treatment Platform for Bladder Cancer
SHANGHAI, May 18, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced a strategic collaboration with UroViu C...
Kapruvia® (difelikefalin) recommended by England's NICE for the treatment of adults with moderate-to-severe CKD-associated pruritus
Recommendation will enable eligible patients in England, Wales and Northern Ireland to access the first licensed treatment for moderate-to-severe chronic kidney disease (CKD)-associated pruritus in adult patients on haemodialysis ST. GALLEN, Switzerland and STAMFORD, Conn., May 18, 2023 /PRNewswi...
Antengene Announces Clearance of U.S. IND for the Phase I Trial of First-in-Class Anti-CD24 Monoclonal Antibody ATG-031
* ATG-031, discovered and developed in-house by Antengene, is the world's first anti-CD24 antibody to advance to the clinic in oncologyand Antengene's third drug candidate to enter clinical studies in the U.S. * The Phase I "PERFORM" study will evaluate the safety and tolerability, pharmacolo...
Antengene Announces Clearance of U.S. IND for the Phase I Trial of First-in-Class Anti-CD24 Monoclonal Antibody ATG-031
- ATG-031, discovered and developed in-house by Antengene, is the world's first anti-CD24 antibody to advance to the clinic in oncologyand Antengene's third drug candidate to enter clinical studies in the U.S. - The Phase I "PERFORM" study will evaluate the safety and tolerability, pharmacolog...
Sirnaomics to receive HK$8 million subsidy from the HKSTP Clinical Translational Catalyst Plan
HONG KONG and GERMANTOWN, Md. and SUZHOU BIOBAY, China, May 17, 2023 /PRNewswire/ -- Sirnaomics Ltd. (the "Company"; together with its subsidiaries, the"Group" or "Sirnaomics"; stock code: 2257), a leading biopharmaceutical company engaging in discovery and development of advanced RNAi therapeuti...
Skyline Therapeutics Brings Multiple Data Presentations to ASGCT 2023
SHANGHAI, May 17, 2023 /PRNewswire/ -- Skyline Therapeutics, an innovation-driven gene therapy company dedicated to developing unique and novel solutions to address unmet needs in rare and severe diseases, is showcasing multiple data presentations at the American Society of Gene and Cell Therapy ...
Inclusion of Recbio In the MSCI China Small Cap Index
TAIZHOU, China, May 17, 2023 /PRNewswire/ -- Jiangsu Recbio Technology Co., Ltd. (the "Company", together with its subsidiaries, the "Group") is pleased to announce that, MSCI announced the results of the semi-annual review of the MSCI Global Small Cap Indexes onMay 12, 2023, and the Company has ...
Antengene Announces NDA Submission for XPOVIO® in Indonesia
SHANGHAI and HONG KONG, May 17, 2023 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hema...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00TAILG's First Flagship Store in Indonesia Grandly Opens
[Picked up by 283 media titles]
2024-11-22 21:59MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00