Biotechnology
CBC GROUP AND MUBADALA CO-LEAD US$315 MILLION FUNDRAISING ROUND FOR HASTEN BIOPHARMA
Proceeds raised will be used to fund future acquisitions, and fuel business development of innovative pipeline assets SINGAPORE, April 20, 2023 /PRNewswire/ -- CBC Group ("CBC"), Asia's largest healthcare-dedicated investment firm headquartered inSingapore, and Hasten Biopharmaceutic Company Lim...
Innovent Announces Overall Survival Results of Phase 2 Study of Pemazyre® (pemigatinib) in Chinese Patients with Advanced Cholangiocarcinoma Presented at AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 19, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
Innovent Announces Overall Survival Results of Phase 2 Study of Pemazyre® (pemigatinib) in Chinese Patients with Advanced Cholangiocarcinoma Presented at AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 20, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
Ajinomoto Bio-Pharma Services Receives FDA Approval for High Potency Fill Line
SAN DIEGO, April 19, 2023 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce that the United States Food and Drug Administration (FDA) has approved the company's high pot...
Nona Biosciences and Washington University Join Forces to Tackle Emerging RNA Viruses
CAMBRIDGE, Mass., April 18, 2023 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited, committed to cutting edge technology innovation and provider of integrated solutions from "Idea to IND" (I to ITM), announced today it has entered into a collaboration agreement w...
AACR 2023 | ImmVira presented preclinical study results of first CAR-T enabler oncolytic product MVR-T7011 at AACR Annual Meeting
SHENZHEN, China, April 17, 2023 /PRNewswire/ -- On April 18, 2023, U.S. time,
ImmVira presented preclinical study results of CAR-T enabler oHSV product
MVR-7011 through poster publication at the American Association for Cancer
Research ("AACR") annual meeting.
Bridge Biotherapeutics Releases Updated Preclinical Data for BBT-207 at the AACR Annual Meeting
* New preclinical data validate anti-tumor efficacy and intracranial activity of the drug candidate * Bridge submitted an IND application to the U.S. Food and Drug Administration in March, and expects to initiate the first-in-human study of BBT-207 this year SEONGNAM, South Korea and CAMBRIDG...
South Korean Biotech, ToolGen Leads the Development of Numerous Cutting-Edge Genome Editing Techniques
SEOUL, South Korea, April 18, 2023 /PRNewswire/ -- Until now, most of the major
breakthroughs in the CRISPR field have been led by US academics. Not anymore;
Toolgen, aSouth Korea-based biotech company, has reported three breakthrough
techniques in top-tier scientific journals.
Technoderma Medicines Initiates TDM-105795 Androgenetic Alopecia Phase 2 Clinical Trial
CHENGDU, China, April 18, 2023 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report that the Company has begun dosing patients in its Androgenetic Alopecia (AGA) Phase 2 clinical trial (NCT05802173) of topical TDM-105795 sol...
Biosyngen announces FDA IND approval of its second product for EBV-positive lymphoma
GUANGZHOU, China, April 18, 2023 /PRNewswire/ -- April 15, 2023, Biosyngen Pte Ltd (hereinafter as "Biosyngen") announced that the Company received IND approval for its second product in the pipeline, a T-cell redirection therapy for the treatment of EBV-positive lymphoma. A week prior to this, t...
YS Biopharma Announces Record Vaccine Revenues and Preliminary Financial Results for FY2023 Ended March 31, 2023
FY2023 Revenues Increased by Approximately 30% Year Over Year GAITHERSBURG, Md., April 18, 2023 /PRNewswire/ -- YS Biopharma Co., Ltd. (NASDAQ: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and commercializing new ...
Daewoong Pharmaceutical and Sygnature Discovery announce drug discovery research collaboration
- 'Daewoong Pharmaceutical enters research collaboration to discover a novel small molecule to target autoimmune diseases using fragment-based drug discovery and virtual high throughput screening SEOUL, South Korea and NOTTINGHAM, England, April 18, 2023 /PRNewswire/ -- Daewoong Pharmaceuti...
GC Biopharma celebrates 'World Hemophilia Day'
* To display a large media façade on its Yong-in R&D Center YONGIN, South Korea, April 17, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a leading provider of biopharmaceutical products inSouth Korea, takes part in a campaign to celebrate World Hemophilia Day. The company announced that a l...
Innovent Releases Final Analysis Results of ORIENT-16: the Phase 3 Study of Sintilimab in Combination with Chemotherapy for the First-Line Treatment of Gastric Cancer at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 17, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
Innovent Releases Final Analysis Results of ORIENT-16: the Phase 3 Study of Sintilimab in Combination with Chemotherapy for the First-Line Treatment of Gastric Cancer at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 18, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
Ocedurenone: A Novel Therapy for Uncontrolled Hypertension in Advanced Chronic Kidney Disease
PRINCETON, N.J., April 18, 2023 /PRNewswire/ -- EMJ Nephrology interviewed two World-renowned experts in nephrology and cardiology,George Bakris and Faiez Zannad, regarding the challenges of treating uncontrolled hypertension (uHTN) in patients with advanced chronic kidney disease (CKD), a large ...
Innovent Announces Updated Data of Phase 1 Clinical Trial for IBI351 (KRAS[G12C] Inhibitor) as Monotherapy for Solid Tumors at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 18, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmolog...
Shanghai Cellular Biopharmaceutical Group presents first data from Phase I trial evaluating a novel CAR-T in advanced liver cancer at AACR Annual Meeting
Promising anti-tumor activity reported for C-CAR031, a novel Glypican 3 (GPC3)-targeting cell therapy designed by AstraZeneca SHANGHAI, April 18, 2023 /PRNewswire/ -- Shanghai Cellular Biopharmaceutical Group Ltd. (the Company, or Shanghai Cellular Bio), a company engaged in the drug development...
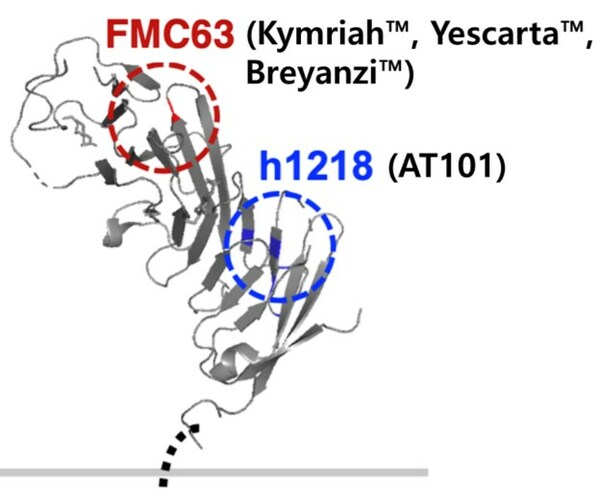
Promising Efficacy of AT101 CAR-T for Blood Cancer
SEOUL, South Korea, April 17, 2023 /PRNewswire/ -- AbClon, a South Korean biotech firm, presented non-clinical and phase 1 interim results of its AT101 novel CAR-T therapy at the annual 2023 AACR conference. AT101 targets the CD19 protein for treatment of blood cancer. AT101 demonstrated superio...
EXCHANGE OFFER DECLARED UNCONDITIONAL
KAISERAUGST, Switzerland, HEERLEN, Netherlands and GENEVA, April 18, 2023 /PRNewswire/ -- THIS PRESS RELEASE IS NOT FOR GENERAL RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, IN OR INTO, DIRECTLY OR INDIRECTLY,THE UNITED STATES OR ANY OTHER JURISDICTION WHERE SUCH RELEASE, PUBLICATIO...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00Announcement of "Cyber Sousa Award" Winners of the 16th Xiamen International Animation Festival
[Picked up by 272 media titles]
2024-11-26 16:48