Biotechnology
Transcenta was Selected to Present the Preclinical Data of TST004 at the 2022 ISN Frontiers Meetings of Complement Related Kidney Diseases
SUZHOU, China, June 28, 2022 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, announces that it was selected ...
BGI Group Continues To Lead In Life Sciences 22 Years After The Human Genome Project
SHENZHEN, China, June 27, 2022 /PRNewswire/ -- BGI Group continues to lead in life sciences, making breakthroughs from single cell technology to spatiotemporal genomics, 22 years after participating in the Human Genome Project (HGP). BGI was formed to be a part of the HGP, contributing to the se...
Abcam: Appointment of Vice President of Investor Relations
CAMBRIDGE, England, June 27, 2022 /PRNewswire/ -- Abcam
Antengene Announces Clinical Trial Collaboration with BeiGene to Evaluate Selinexor in Combination with Tislelizumab in T and NK-Cell Lymphoma
- Selinexor is an oral small molecule XPO1 inhibitor; tislelizumab is an anti-PD-1 checkpoint inhibitor SHANGHAI and HONG KONG, June 27, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutical company dedi...
Congratulations to Dragon Boat Biopharmaceutical from Sanyou Biopharmaceuticals on the NMPA acceptance of the CLDN 18.2/CD47 bsAb clinical trial application
SHANGHAI, June 25, 2022 /PRNewswire/ -- On June 15, 2022, Dragon Boat announced that its IND application of the innovative anti-CLDN 18.2/CD47 bi-specific antibody (bsAb) injection (R&D code: BC007) was officially accepted by National Medical Products Administration (NMPA) under the acceptance nu...
Sanyou Biopharmaceuticals forged strategic partnership with Dragon Sail Pharmaceutical to upgrade integrated innovative antibody drug R&D
SHANGHAI, June 24, 2022 /PRNewswire/ -- Sanyou Biopharmaceuticals, a biological high-tech enterprise focusing on R&D and services of innovative antibody drugs, and Dragon Sail Pharmaceutical, aCDMO and CMO enterprise dedicated to providing world-leading high-end biological drug manufacture servic...
Daewoong Pharmaceutical begins multinational phase 2 clinical trial for DWN12088, a new drug for idiopathic pulmonary fibrosis
- U.S. Food and Drug Administration (FDA) approved the IND for the phase 2 clinical trial for patients with idiopathic pulmonary fibrosis - Daewoong Pharmaceutical to start a multinational phase 2 clinical trial for DWN12088 in September SEOUL, South Korea, June 24, 2022 /PRNewswire/ -- Daewoong...
Novavax COVID-19 Vaccine Nuvaxovid™ Recommended for Expanded Conditional Marketing Authorization in the European Union by CHMP for Adolescents Aged 12 Through 17
* Upon authorization, Nuvaxovid™ would be the first protein-based option for adolescents aged 12 through 17 inEurope * Nuvaxovid™ demonstrated 80% efficacy and was generally well-tolerated in adolescents GAITHERSBURG, Md., June 24, 2022 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechn...
Use of BioMAP Platform to Assess Nutraceuticals Published in BioMed Central
ST. CHARLES, Mo., June 24, 2022 /PRNewswire/ -- Eurofins Discovery, the leading brand with over 35 years of success providing a complete solution of products and services for drug discovery, announces a client publication advancing cognitive health research through use of the company's BioMAP...
MGI Wins Company Innovation of the Year at 2022 Globee® Awards for Advanced COVID-19 Response Efforts
SHENZHEN, China, June 24, 2022 /PRNewswire/ -- MGI, a company committed to being a world-leading life science innovator, has been awarded a Silver Globee® in the 14th Annual 2022 Golden Bridge Business and Innovation Awards® for its innovative COVID-19 pandemic control and response efforts worldw...
Alterity Therapeutics Announces Regulatory Authorization to Proceed with ATH434 Phase 2 Clinical Trial in Italy
MELBOURNE, Australia and SAN FRANCISCO, June 23, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative diseases, today announced that the Italian Medicin...
Berry Oncology launches HIFI Pan-Cancer Screening, a multi-cancer early screening product for detecting six high-risk cancers at one time
BEIJING, June 23, 2022 /PRNewswire/ -- On June 23, Berry Oncology, a global leading company specialized in genomic testing and early cancer screening, announced the launch of its innovative one-time precision product HIFI Pan-Cancer Screening, which is an early multi-cancer screening product ...
Viva Biotech Successfully Held The 3rd Annual Partnership Summit
SHANGHAI, June 23, 2022 /PRNewswire/ -- June 16th-20th, 2022 (Beijing time), Viva Biotech 2022 Partnership Summit was successfully held. Over 300 attendees joined the Summit, including founders from portfolio companies, representatives from global investment institutions, R&D heads from pharmaceu...
I-Mab Receives Top Rankings in Five Categories by Institutional Investor
GAITHERSBURG, Md. and SHANGHAI, June 23, 2022 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it was ranked among the top companies i...
Hyundai Mobis plans to extend biotech to mobility to take care of the driver's health
* The new healthcare tech 'Smart Cabin' controller that analyzes vital signs, such as posture, heart rate, and brainwaves, to help with safe driving. * Switches to autonomous driving when the driver is found to be stressed and manages the CO2 level in the cabin * Embedded technology that app...
Mojia Biotech Completes Series B Financing to Advance Manufacturing of Bio-based Materials
SHANGHAI, June 22, 2022 /PRNewswire/ -- Mojia Biotech, a Shanghai-based leading bio-manufacturing company dedicated to sustainable development and carbon neutrality, announced the completion of an $80 million Series B Financing. The funds will be used to commercialize its Viridimin™ brand of anim...
Sirnaomics to Present the Latest Developments of Dual-Targeted RNAi Therapeutics based on its Proprietary GalAhead™ Program at the 4th Annual RNA Therapeutic: From Concept to Clinic Symposium
GAITHERSBURG, Md. and SUZHOU, China, June 22, 2022 /PRNewswire/ -- Sirnaomics Ltd. (the "Company" or "Sirnaomics", stock code: 2257.HK), a leading biopharmaceutical company in discovery and development of RNAi therapeutics, announced that the Company's Chief Technology Officer,Dmitry Samarsky, Ph...
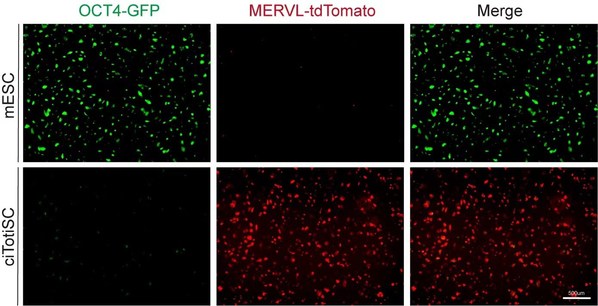
Scientists take the first step to master an all-powerful cell type in the beginning of life
Sheng Ding and his team at Tsinghua University School of Pharmaceutical Sciences publish innovative work in Nature BEIJING, June 21, 2022 /PRNewswire/ -- From cloning to regeneration, how to find alternative paths to create or rejuvenate life has been one of the big questions for biologists. It ...
3Z closes $2 million funding for preclinical development of novel ADHD and Insomnia therapeutics identified by 3Z´s zebrafish drug discovery platform
REYKJAVIK, Iceland, June 20, 2022 /PRNewswire/ -- 3Z, a Reykjavik based drug discovery company is pleased to announce the closure of a$2 million funding round led by seasoned investors in pharmaceuticals and medical technology. The just closed funding will accelerate finalization of preclinical s...
Acepodia Announces FDA Clearance of IND Application for ACE1831, an Anti-CD20 Armed Allogeneic gamma delta T-cell Therapy Candidate to Treat Patients with non-Hodgkin's Lymphoma
ACE1831 is a potential antibody‑armed allogeneic gamma delta T cell therapy developed using Acepodia's unique antibody-cell conjugation (ACC) technology as an optimized T cell engager platform to treat patients with non-Hodgkin's lymphoma ALAMEDA, Calif. and TAIPEI, Taiwan, June 20, 2022 /PRNews...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 295 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 292 media titles]
2024-11-26 13:00