Biotechnology
Amaroq Therapeutics hits early milestones and makes key appointments to Scientific Advisory Board
Promising 'dark matter' cancer therapy has clinical trials in sight DUNEDIN, New Zealand, June 8, 2022 /PRNewswire/ -- Amaroq Therapeutics, a biotechnology company focussed on developing a new class of therapeutics that target lncRNA in cancer, is progressing towards clinical trials following a ...
Medison Pharma Announces the Approvals by Health Canada and Therapeutics Goods Administration of KIMMTRAK® (tebentafusp) for the Treatment of Unresectable or Metastatic Uveal Melanoma
KIMMTRAK has been approved in Canada and Australia for the treatment of an
aggressive form of ocular melanoma and follows approval inthe United States and
the European Union
PETACH TIKVAH, Israel, June 8, 2022 /PRNewswire/ -- Today, Medison Pharma
OnCusp Therapeutics and Multitude Therapeutics Enter into an Ex-China Licensing Agreement for a Potentially Highly Differentiated CDH6-Targeting Antibody Drug Conjugate
NEW YORK, June 8, 2022 /PRNewswire/ -- OnCusp Therapeutics announced today a licensing agreement with Multitude Therapeutics for the development and commercialization of AMT-707 (now referred to as CUSP06), a potentially highly differentiated second-in-class CDH6 antibody drug conjugate ("ADC"). ...
FDA Advisory Committee Recommends Emergency Use Authorization of Novavax COVID-19 Vaccine for People Aged 18 Years and Older
* Novavax COVID-19 vaccine receives positive vote from U.S. Food and Drug Administration Vaccines and Related Biological Products Advisory Committee * If Emergency Use Authorization is granted by the FDA, the Novavax COVID-19 vaccine would become the first protein-based COVID-19 vaccine availa...
Antengene Announces First Patient Dosed in the Phase I STAMINA-001 Study of ATG-037 for the Treatment of Patients with Locally Advanced or Metastatic Solid Tumors
SHANGHAI and HONG KONG, June 8, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class therapeutics in hema...
First Patient Enrolled in SELUTION SLR IDE BTK Study
LEIPZIG, Germany, June 8, 2022 /PRNewswire/ -- The first patient has been
enrolled in the FDA IDE BTK (Below-the-Knee) SELUTION4BTK clinical trial
involving SELUTION SLR™, MedAlliance's novel sirolimus-eluting balloon, just
one week after receiving IDE approval.
Nature Scientific Reports to publish novel decentralized federated AI learning algorithm that connects globally distributed data silos containing private and poor-quality data
SAN FRANCISCO, June 7, 2022 /PRNewswire/ -- Nature Scientific Reports journal has published results of a new decentralized federated AI learning algorithm from Presagen that can train AI on data that is distributed globally, without having to move private data to a central location. The breakthro...
The 3rd BIO International Convention 2022 is Approaching
SAN DIEGO, June 7, 2022 /PRNewswire/ -- With the 3rd BIO International Convention 2022 soon approaching, Neurophth Therapeutics is looking forward to discussing collaboration opportunities in gene therapy for both rare and common ocular diseases. Neurophth Therapeutics is a leading clinical-stag...
PHASE II STUDY OF PAXALISIB IN BRAIN METASTASES ADVANCES TO EXPANSION STAGE IN BREAST CANCER BRAIN METASTASES COHORT
SYDNEY, June 7, 2022 Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that a phase II, genomically-guided study of multiple therapies in patients with brain metastases, led by the Alliance for Clinical Trials in Oncology (NC...
Molecular Stethoscope, Inc. announces new investment from the Alzheimer's Drug Discovery Foundation
Investment by the ADDF will accelerate the translation of Molecular Stethoscope's cf-mRNA liquid biopsy technology platform into products and services for the diagnosis and management of Alzheimer's disease SOUTH SAN FRANCISCO, Calif., June 7, 2022 /PRNewswire/ -- Molecular Stethoscope, a leadin...
Chordia Therapeutics Announces Interim Results of the Phase 1 Clinical Trial of CLK Inhibitor CTX-712 at the 2022 ASCO Annual Meeting
KANAGAWA, Japan, June 7, 2022 /PRNewswire/ -- Chordia Therapeutics Inc. ("Chordia"), a biotech company engaged in the research and development of novel therapies for cancers, today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712, a selective pan-CDC-...
Chordia Therapeutics Announces Interim Results of the Phase 1 Clinical Trial of CLK Inhibitor CTX-712 at the 2022 ASCO Annual Meeting
KANAGAWA, Japan, June 7, 2022 /PRNewswire/ -- Chordia Therapeutics Inc. ("Chordia"), a biotech company engaged in the research and development of novel therapies for cancers, today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712, a selective pan-CDC-...
SCG Cell Therapy And A*STAR's IMCB Collaborate To Accelerate Clinical Translation of Immune Cell-based Therapy
* The collaboration agreement works toward accelerating the study of SCG's immunotherapy pipeline and candidates such as CAR-T, TCR-T therapies, antibodies, and vaccines. * SCG will contribute its proprietary technologies to the therapeutic development of immune cell-based therapy candidates....
Antengene Announces HREC Approval in Australia for the Phase I Trial of the Small Molecule ATR Inhibitor ATG-018
– Discovered in-house by the internal R&D Team at Antengene, ATG-018 is an orally-bioavailable, small molecule ataxia telangiectasia and Rad3-associated (ATR) kinase inhibitor that targets the DNA damage response (DDR) pathway. – This Phase I study will evaluate the safety, pharmacology and prelim...
Pharmactive Enters Strategic Partnership with Nutraconnect for APAC
Pharmactive targets Asia Pacific Market with new collaboration
MADRID, June 7, 2022 /PRNewswire/ -- Pharmactive Biotech Products
iNtRON Completes GLP-TOX Studies of BAL200
* A novel drug candidate for anthrax received orphan drug designation (ODD) by the US FDA. BOSTON and SEOUL, Korea, June 6, 2022 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON" or "Company") announced today that the company has successfully completed the GLP toxicology studies of BAL200. The co...
Insilico Medicine Raises $60 Million in Series D Financing to Advance Pipeline and Launch AI-powered Drug Discovery Robotics Laboratory
NEW YORK, June 6, 2022 /PRNewswire/ -- Insilico Medicine, a clinical-stage end-to-end artificial intelligence (AI)-driven drug discovery company, announced today that it has completed a$60 million Series D financing from a syndicate of global investors with expertise in investing in the biopharm...
Senhwa's Pindnarulex in Combination Study with Pfizer's Talazoparib for the Treatment of Prostate Cancer Granted Approval to Initiate from Australian HREC
TAIPEI and SAN DIEGO, June 6, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that it has received written approval from the Human Research Ethics Committ...
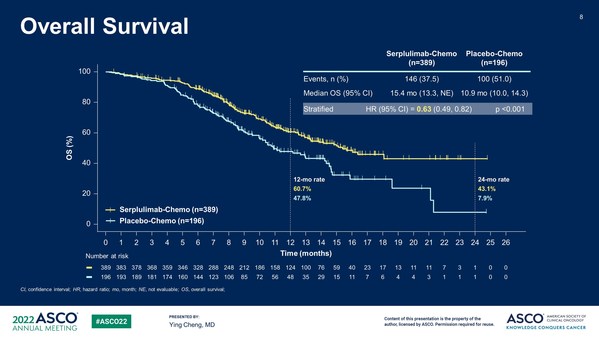
ASTRUM-005: Henlius Released Phase 3 Study Results for the First-line Treatment of Small Cell Lung Cancer of Serplulimab at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that an international randomized phase 3 study (ASTRUM-005) of HANSIZHUANG (serplulimab), an anti-PD-1 mAb independently developed by Henlius, as first-line treatment for extensive-stage small-cell lung can...
CARsgen Therapeutics Presents Updated Data for CT041 Claudin18.2 CAR T-cells in Solid Tumors at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that at the 2022 American Society of Clinical Oncology (ASCO) Annual M...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 294 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 291 media titles]
2024-11-26 13:00