Health Care/Hospital
PharmAbcine Unveils Olinvacimab's Positive Results from Phase Ib Combination Studies at KSMO 2020
DAEJEON, South Korea, Sept. 14, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) today announced positive data from its two combination trials of olinvacimab, its leading clinical candidate in oncology, with MSD's pembrolizumab at the 13th Annual Meeting of the Korean Society of Medical O...
Ascentage Pharma's MDM2-p53 Inhibitor APG-115 Granted Orphan Drug Designation by the FDA for the Treatment of Gastric Cancer
SUZHOU, China and ROCKVILLE, Md., Sept. 14, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the US Food and Drug ...
Innovent and Lilly Jointly Announce Results of Six Clinical Studies of TYVYT® (sintilimab injection) to be Presented at the European Society for Medical Oncology (ESMO) Virtual Congress 2020
SAN FRANCISCO and SUZHOU, China, Sept. 14, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for treatment of oncology, metabolic, autoimmune and other major disea...
Countering counterfeit medicines in the fast-paced world of e-commerce
BANGKOK, Sept. 14, 2020 /PRNewswire/ -- Research shows that counterfeit medicine cannot cure illnesses and can cause moreharm to your health. Counterfeit medicines have been in the market for a long time, and without sufficient awareness, consumption of these substances can lead to unexpected sy...
Merck Advances Oncology Portfolio and Pipeline with New and Long-term Data in Multiple Cancers at ESMO 2020
* New analyses from Phase III JAVELIN Bladder 100 study of BAVENCIO®* assess efficacy across subgroups, patient-reported outcomes and exploratory biomarkers in advanced urothelial cancer * Overall efficacy data, and analyses of brain metastases and HRQoL for tepotinib† from largest ongoing st...
Global Fund Partnership Has Saved 38 Million Lives - but COVID-19 Could Wipe Out Progress
GENEVA, Sept. 14, 2020 /PRNewswire/ -- A new report by the Global Fund to Fight AIDS, Tuberculosis and Malaria is a call to action to urgently invest to protect decades of progress against HIV, TB and malaria that are being derailed as a knock-on effect of the COVID-19 pandemic. According to ...
Gannex Filed US IND for Its NASH Drug ASC42, an FXR Agonist
SHANGHAI, Sept. 14, 2020 /PRNewswire/ -- Gannex Pharma Co., Ltd., a wholly owned company of Ascletis Pharma Inc. (HKEX:1672) announces today that it has filed investigational new drug application (IND) with US FDA for its non-alcoholic steatohepatitis (NASH) drug candidate ASC42. ASC42 is an in-...
Virtual reality startup and Fujitsu bring immersive drug design software to Japanese market
SAN DIEGO, Sept. 14, 2020 /PRNewswire/ -- San Diego–based virtual reality (VR) startup Nanome, Inc. has entered into an agreement with Fujitsu to bring their signature product—an immersive scientific design and collaboration platform—to the Japanese market. The software is already used by more th...
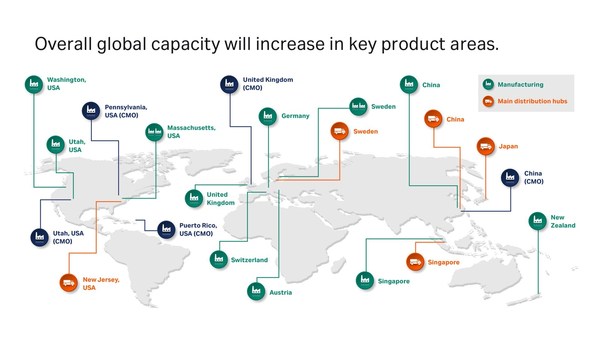
5 years, 500 million USD, and nearly 1,000 people: Cytiva invests for global capacity expansion
* Total planned investment is around 500 million USD over five years to raise manufacturing capacity * Continues long-term strategy of increasing capacity to respond to growing industry demand and new market opportunities * Cytiva hiring nearly 1,000 personnel in Austria, China, Singapore, S...
Coronavirus Breakthrough: Senhwa Reports First eIND Silmitasertib Treated Severe COVID-19 Patient - Discharged Following Five Days of Treatment
TAIPEI and SAN DIEGO, Sept. 11, 2020 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a clinical-stage biopharmaceutical company focused on next generation DNA Damage Response (DDR) therapeutics for the treatment of cancer, announced today that the first patient with severe COVID-19 demonst...
Haoma Medica Announces Plenary Presentation for NaQuinate, a Potential Novel Treatment for Osteoporosis, at ASBMR 2020 Annual Meeting
LONDON, Sept. 12, 2020 /PRNewswire/ -- Haoma Medica announced a presentation made today at the 2020 American Society for Bone and Mineral Research: Dr Andrew Pitsillides, Professor of Skeletal Dynamics at the Royal Veterinary College, London presented 'NaQuinate: A Drug that Selectively Synergize...
SK Biopharmaceuticals Initiates Clinical Development Program for Cenobamate in Asia
Phase 3 clinical trial to support registrational filings in China and Japan Company on track to expand presence in Asia, reflecting its commitment to reach more people with epilepsy worldwide PANGYO, GYEONGGI PROVINCE, Korea, Sept. 12, 2020 /PRNewswire/ -- SK Biopharmaceuticals, Co., Ltd., a glo...
Harbour BioMed and Hualan Genetic Announced Collaboration to Develop Multiple Innovative Antibody Programs
CAMBRIDGE, Mass., ROTTERDAM, Netherlands, SUZHOU,China and XINXIANG, China, Sept. 11, 2020 /PRNewswire/ -- Harbour BioMed (HBM), a global, clinical-stage, innovative biopharmaceutical company today announced a strategic partnership agreement with Hualan Genetic Engineering Co., Ltd (Hualan Geneti...
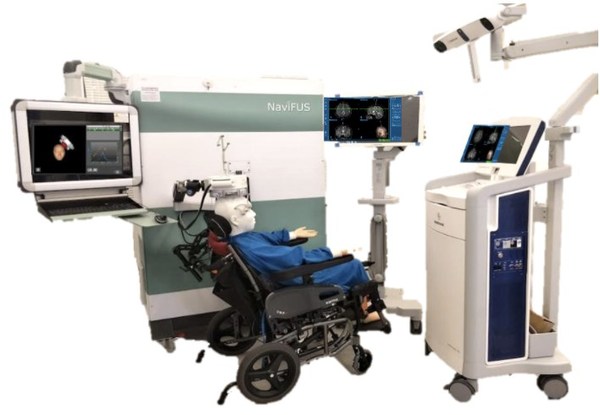
NaviFUS Launches Neuronavigation-guided Clinical Trial to Open the Blood-Brain-Barrier for Combination FUS-Bevacizumab Therapy in rGBM Patients
TAIPEI, Sept. 11, 2020 /PRNewswire/ -- Genovate Biotech (TPEX:4130) subsidiary andTaiwan-based focused ultrasound (FUS) manufacturer NaviFUS Corporation is pleased to announce the start of its clinical trial (NCT04446416) for the combination of FUS plus bevacizumab therapy. Researchers at Linkou ...
New MAVENCLAD® Data at ACTRIMS-ECTRIMS MSVirtual2020 Meeting Highlight Rapid Onset of Action and Compelling Post-Approval Safety
In MAGNIFY-MS, patients experienced a rapid onset of action from end of Month 1 that was significant in all study periods versus baseline Post-approval safety analysis showed no increased risk of viral respiratory infections and lower rates of malignancy than in the clinical trial program Data f...
Booming Demand for Innodisk's CAN Bus Solutions Amid Pandemic and Autonomous Vehicle Trend
TAIPEI, Sept. 11, 2020 /PRNewswire/ -- Innodisk's CAN bus expansion modules are taking the world by storm. Unprecedented demand for autonomous vehicle technology amid the ongoing COVID-19 pandemic and continued growth of industrial automation and smart healthcare mean that Innodisk's industry-le...
The Air Liquide Foundation supports 10 scientific projects and 21 social emergency aid projects in the context of its Covid-19 Initiative
PARIS, Sept. 11, 2020 /PRNewswire/ -- In response to the Health crisis, the Air Liquide Foundation launched as early asMarch 2020 the Covid-19 Initiative. More than 2million euros have been mobilized over two years, with a double objective: to support scientific research projects and to reinforce...
Sai Life Sciences expands suite of cellular analysis platforms in Cambridge, MA
CAMBRIDGE, Mass. and HYDERABAD, India, Sept. 11, 2020 /PRNewswire/ -- Sai Life
Sciences, one ofIndia's fastest growing Contract Development & Manufacturing
Organizations (CDMOs)
The Korea Ginseng Association, announces new direction for Korean ginseng, a publicly listed functional ingredient for bone health
SEOUL, South Korea, Sept. 11, 2020 /PRNewswire/ -- The Korea Ginseng Association announced that Korean ginseng is registered as a 'publicly listed functional ingredient for bone health" by the Ministry of Food and Drug Safety (MFDS) last July. With this news, development of various health functio...
Pharmalutions And Movilitas.Cloud Deliver Serialization Solutions for the Asia-Pacific Region
COLUMBIA, Md., Sept. 11, 2020 /PRNewswire/ -- Movilitas announced Pharmalutions Pte Ltd as its newest Movilitas.Cloud partner for theAsia-Pacific region. Under the partnership, Pharmalutions provides greater access to compliance-ready solutions with Movilitas.Cloud, a GAMP 5 validated software as...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00Announcement of "Cyber Sousa Award" Winners of the 16th Xiamen International Animation Festival
[Picked up by 272 media titles]
2024-11-26 16:48