Medical/Pharmaceuticals
Mayapada Healthcare Ready to Serve PCR Swab Covid-19
JAKARTA, Indonesia, May 28, 2020 /PRNewswire/ -- Mayapada Healthcare is ready to serve the PCR Swab Test in all Mayapada Hospital units. Supported by specialized Biomolecular Laboratory, certified Bio Safety Level 2 (BSL2), PCR equipment with the latest technology, and experts in their fields, Ma...
Shanghai Cell Therapy Group Launches Collaboration with USC researcher to Improve the ex vivo Expansion of Hematopoietic Stem Cells for Clinical Applications
SHANGHAI, May 27, 2020 /PRNewswire/ -- Shanghai Cell Therapy Group (SHCell) recently entered into a six-year research collaborative project with Professor Qi-Long Ying from theUniversity of Southern California (USC). Through the project, sponsored by$3.6 million from the Baize Plan Fund, the Ying...
Yuyu Pharma Signs Contract for Baseball Field Ad Campaign
SEOUL, South Korea, May 28, 2020 /PRNewswire/ -- Yuyu Pharma
I-Mab Reports Interim Results from Part 1 Study for Anti-GM-CSF Antibody TJM2 to Treat COVID-19 Patients with Cytokine Release Syndrome
SHANGHAI and GAITHERSBURG, MD, May 27, 2020 /PRNewswire/ -- I-Mab (NASDAQ: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced interim results from a multi-center, double blinded, randomized, placeb...
Transcenta Presents First Data from Phase 1 Study of pH-Dependent PDL1 Antibody MSB2311 in Patients with Pre-treated Advanced Solid Tumors and Select Hematological Malignancies
SUZHOU, China, May 27, 2020 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta"), a global biotherapeutics company with fully-integrated capabilities in discovery,development and manufacturing of antibody-based therapeutics, announced today results reported for the first time from a Phase 1...
Merck and Baylor College of Medicine Collaborate to Advance a Vaccine Manufacturing Platform to Fight Covid-19
-- Company will focus on process development and improvements for manufacturing Baylor's Covid-19 vaccine candidates DARMSTADT, Germany, May 27, 2020 /PRNewswire/ -- Merck, a leading science and technology company, andBaylor College of Medicine, Houston, Texas, USA, today announced an extensio...
ActLight Signs an Agreement on Single Photon Sensitivity Technology With Leading Sensors Company
LAUSANNE, Switzerland, May 27, 2020 /PRNewswire/ -- ActLight, the Swiss technology firm known for its best in class signal-to-noise ratio photodiodes, announced today that it has signed a service agreement based on its Single Photon Sensitivity technology with a leading company in the sensors mar...
Ajinomoto Bio-Pharma Services Signs Manufacturing Agreement with Humanigen for Lenzilumab, Currently in FDA-Approved Phase III Study for COVID-19
SAN DIEGO, May 27, 2020 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce it has entered into a manufacturing agreement with Humanigen, Inc., for the fill finish supply ...
Daewoong Pharmaceutical Acquires Halal Certification for 'Easyef' through Daewoong Infion
SEOUL, South Korea and JAKARTA, Indonesia, May 27, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (CEOSengho Jeon) announced on May 25 that the Indonesian joint venture Daewoong Infion (CEOChang Woo Suh) obtained Halal certification for the diabetic foot ulcer drug 'Easyef topical solution(Easyef)...
DERMALOG Fever Check at German Baltic Hotel
USEDOM and HAMBURG, Germany, May 27, 2020 /PRNewswire/ --
- Picture is available at AP Images (http://www.apimages.com
Orteq® Sports Medicine Announces Publication of 5-year Multi-Center Clinical Data for the Actifit® Meniscal Scaffold in the American Journal of Sports Medicine (AJSM), Receives FDA Breakthrough Device Designation
LONDON, May 26, 2020 /PRNewswire/ -- Orteq Sports Medicine Ltd. (www.orteq.com ), a developer of joint preservation solutions for orthopedic patients, announces 5-year, multi center, peer-reviewed data published in the AJSM analyzing the Actifit meniscal scaffold that shows more than 87% survival ...
United Imaging uMR OMEGA Cleared by FDA, Available in U.S. Market
HOUSTON, May 26, 2020 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, announced U.S. Food & Drug Administration (FDA) clearance of the uMR OMEGATM magnetic resonance imaging (MRI) scanner. The uMR OMEGA offers the world's first ultra-wide 75...
EDAN CEO: Mushroomed under the Crisis
SHENZHEN, China, May 26, 2020 /PRNewswire/ -- A critical medical device provider of the COVID-19 pandemic, EDAN, supplied hundreds of thousands of medical devices toGermany, Spain, Italy, the United Kingdom, the United States, Russia, Mexico, Brazil, and many other countries. Hao Zhang, the CEO o...
Polygiene Treating Respirators From Carbonado With ViralOff
- Indian brand Carbonado just launched AerFit NEO respirators with Polygiene ViralOff® textile treatment technology STOCKHOLM, May 26, 2020 /PRNewswire/ -- The company will place orders in volumes of 500,000 to one million (1,000,000) respirators in the coming three to six months and has a broad...
AFT Pharmaceuticals Donates Almost $110,000 To Health Charities When They Need It Most
SYDNEY, May 26, 2020 /PRNewswire/ -- Three health charities have been given a much-needed boost at a time when the charity sector, which relies heavily on income from public events and other in-person fundraising initiatives, is being impacted by the COVID-19 pandemic. Starlight Children's Founda...
Nucleus Network commences Novavax' Phase 1/2 COVID-19 vaccine trial in Australia
MELBOURNE, Australia, May 26, 2020 /PRNewswire/ -- Australia's largest Phase 1 Clinical trials specialist, Nucleus Network, has today commenced dosing the first human participants for the Phase 1/2 trial of NVX‑CoV2373, a SARS-CoV-2 Recombinant Spike Protein Nanoparticle vaccine, at itsMelbourne ...
Innovent Announces First Patient Dosed in A Phase 1 Clinical Trial of Anti-TIGIT Monoclonal Antibody in China
SUZHOU, China, May 26, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic and other major diseases, today anno...
China's medical rehabilitation market likely to see significant growth potential, five key sub-sectors identified, finds KPMG report
HONG KONG, May 25, 2020 /PRNewswire/ -- With increasing importance placed on medical rehabilitation, KPMG has identified five out of eleven rehabilitation sub-sectors with the highest potential in terms of their market attractiveness and market entry abilities. These are neurological rehabilitati...
New Late-Breaking Data at EAN Indicate Evobrutinib is the First BTK Inhibitor to Report Efficacy and Safety in MS Over 108 Weeks
- Not intended for UK and U.S. based media - Investigational evobrutinib is the first and only Bruton's Tyrosine Kinase inhibitor to demonstrate high and sustained efficacy through 108 weeks in clinical studies - No new safety signals identified in the 60 week open-label extension, consistent ...
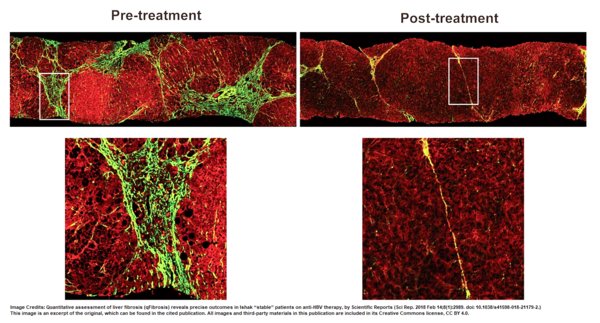
HistoIndex's AI-based Digital Pathology Platform: A Validated Quantification Tool Recommended in the Guidelines for the Prevention and Treatment of Chronic Hepatitis B in China
SINGAPORE, May 25, 2020 /PRNewswire/ -- Singapore medtech company HistoIndex
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00