Medical/Pharmaceuticals
I-Mab Appoints International Gastrointestinal Oncology Expert Dr. Andrew Zhu to its Scientific Advisory Board
SHANGHAI and GAITHERSBURG, Md., June 21, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced the appointment ofAndrew Zhu, MD, PhD, to the Company's ...
Duke-NUS and GenScript Announce Notice of Allowance for U.S. Patent Application for SARS-CoV-2 Surrogate Virus Neutralization Test
PISCATAWAY, N.J. and SINGAPORE, June 21, 2021 /PRNewswire/ -- GenScript Biotech Corporation ("GenScript", Stock Code: 1548.HK), a world-leading life sciences research and application service and product provider, and Duke-NUS Medical School, a premier, research intensive medical school, annou...
RedHill Biopharma Announces Presentation of Positive Oral Opaganib Phase 2 Data in COVID-19
TEL AVIV, Israel and RALEIGH, NC, June 21, 2021 /PRNewswire/ -- RedHill
Biopharma Ltd.
HUTCHMED Announces NMPA Approval of Surufatinib (Sulanda(R) in China) for Advanced Pancreatic Neuroendocrine Tumors
HONG KONG, June 21, 2021 /PRNewswire/ -- HUTCHMED (China) Limited ("HUTCHMED
CStone announces first prescriptions of precision therapy GAVRETO® (pralsetinib) issued across China for the treatment of adults with locally advanced or metastatic RET fusion-positive non-small cell lung cancer after platinum-based chemotherapy
SUZHOU, China, June 21, 2021 /PRNewswire/ -- GAVRETO® (pralsetinib), a precision therapy discovered by CStone Pharmaceuticals' partner Blueprint Medicines, had the first batch of prescriptions issued at Guangdong Province People's Hospital and around 100 other hospitals acrossChina. GAVRETO is al...
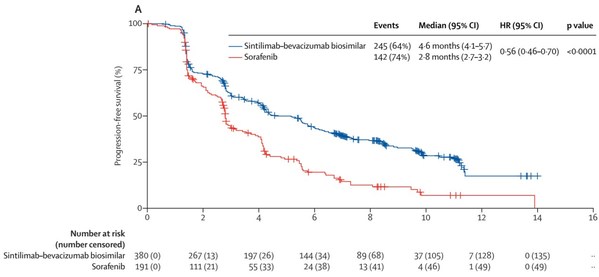
Study Results of Sintilimab in Combination with Bevacizumab Biosimilar IBI305 for the First-Line Treatment of Hepatocellular Carcinoma Published in The Lancet Oncology
SAN FRANCISCO and SUZHOU, China, June 21, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major dise...
EAN Congress: COVID-19 leads to significant cognitive and behavioural problems in patients
VIENNA, June 21, 2021 /PRNewswire/ -- COVID-19 patients suffer from cognitive and behavioural problems two months after being discharged from hospital, a new study presented at the 7th Congress of the European Academy of Neurology has found[1]. Issues with memory, spatial awareness and informati...
Bioheng Biotech received Orphan Drug Designation from the U.S. FDA for the treatment of T-ALL.
NANJING, China, June 20, 2021 /PRNewswire/ -- Nanjing Bioheng Biotech Co., Ltd. Announced UCAR T cell therapy product targeting CD7, code CTD401, received Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) for the treatment of T-cell acute lymphoblastic leukemia (T-ALL...
CARsgen Therapeutics officially listing on HKEX
HONG KONG, June 19, 2021 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited
(the "Company", stock code: 2171.HK) today announced that the Company's Shares
have been traded on the Main Board of The Stock Exchange of Hong Kong Limited
under the stock code "2171.HK".
InnoCare Presents Latest Clinical Data of Orelabrutinib at the16th International Conference on Malignant Lymphoma
BEIJING, June 18, 2021 /PRNewswire/ -- InnoCare (HKEX: 09969), a leading biopharmaceutical company focusing on cancer and autoimmune diseases, announced today the company presented the latest clinical data of the Bruton's tyrosine kinase (BTK) inhibitor orelabrutinib atthe 16th International Conf...
deCODE genetics: Predicting the probability of death
REYKJAVIK, Iceland, June 18, 2021 /PRNewswire/ -- Scientists from deCODE
genetics have developed a predictor based on protein measurements in blood
samples that predicts the time to all-cause death better than traditional risk
factors.
China and India Combat Coronavirus Pandemic Together: Online Ceremony Held for the Handover of Medical Supplies to Aid India
HONG KONG, June 18, 2021 /PRNewswire/ -- On 11 June 2021, an online ceremony was held for the handover of anti-pandemic supplies to aidIndia. Medical supplies toIndia included 135 oxygen concentrators, 5 ventilators and 3,300 KN95 masks. Present at the event were Tang Guocai, Chinese Consul Gener...
Latest testing confirms Viraleze highly effective against Alpha, Beta and Gamma COVID variants
MELBOURNE, Australia, June 18, 2021 /PRNewswire/ -- New data released today
shows thatAustralia's Viraleze™ anti-COVID nasal spray is more than 99.9%
effective against three of the four deadliest variants of the SARS-CoV-2 virus.
Antengene Provides an Update on Its Latest Developments
* Announces two new in-house discovered assets - ATG-031, a potential first-in-class anti-CD24 monoclonal antibody, and ATG-027, a potential first-in-class B7H3/PD-L1 bi-specific antibody * Provides an update on commercial readiness across the Asia Pacific markets for selinexor, Antengene's f...
Latest testing confirms Viraleze highly effective against Alpha, Beta and Gamma COVID variants
MELBOURNE, Australia, June 18, 2021 /PRNewswire/ -- New data released today
shows thatAustralia's Viraleze™ anti-COVID nasal spray is more than 99.9%
effective against three of the four deadliest variants of the SARS-CoV-2 virus.
Genesis Rapidly Expands its Surgical Device Portfolio to Meet Growing Demand
SINGAPORE, June 18, 2021 /PRNewswire/ -- Genesis MedTech Group (Genesis) is pleased to announce it has completed the acquisition of Horcon, aChina-based suture company, and secured exclusive distributorship rights for Sejong Medical's trocar products inChina. This comes on the back of its recent ...
Gilead Sciences Announces Subanalyses of Safety and Efficacy Data from a Phase 2 Study of Chronic Hepatitis B Patients with Renal or Hepatic Impairment Switching to Vemlidy(r)
HONG KONG, June 18, 2021 /PRNewswire/ -- Gilead Sciences, Inc. today announced new sub-analysis data from a Phase 2 open-label study (GS-US-320-4035; NCT03180619), evaluating the safety and efficacy of switching to Vemlidy®# (tenofovir alafenamide 25 mg, TAF) from tenofovir disoproxil fumarate (T...
Nuevocor closes US$24M Series A Financing to Advance Novel Gene Therapies for Cardiomyopathies
SINGAPORE, June 18, 2021 /PRNewswire/ -- Nuevocor, a preclinical-stage biotech company specializing in gene therapy for cardiomyopathies, has announced the completion of an oversubscribed$24 million Series A financing round. The round was co-led by EVX Ventures andBoehringer Ingelheim Venture Fun...
Jacobio Announces First Two Patients Dosed in SHP2 Combination Study, Triggering US$20 Million Milestone Payment
BEIJING and SHANGHAI and BOSTON, June 18, 2021 /PRNewswire/ -- Jacobio Pharmaceuticals (1167.HK) has announced that the first two patients have been dosed in the Phase 1/2a clinical trial ofSHP2 inhibitor JAB-3312 in combination with PD-1 antibody Pembrolizumab and MEK inhibitor Binimetinib respe...
Innovent Announces First Patient Dosed in the Phase II Basket Trial of Taletrectinib for Solid Tumors with NTRK Fusion
SAN FRANCISCO and SUZHOU, China, June 18, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent", HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major disea...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00